
Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
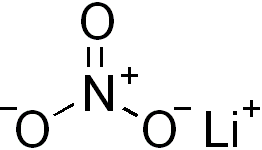
Lithium nitrate is an inorganic compound with the formula LiNO3. It is the lithium salt of nitric acid (an alkali metal nitrate). The salt is deliquescent, absorbing water to form the hydrated form, lithium nitrate trihydrate. Its eutectics are of interest for heat transfer fluids.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O, which is a red-brown deliquescent salt that is soluble in water and other polar solvents.

Mercury(I) nitrate is an inorganic compound, a salt of mercury and nitric acid with the formula Hg2(NO3)2. A yellow solid, the compound is used as a precursor to other Hg22+ complexes. The structure of the hydrate has been determined by X-ray crystallography. It consists of a [H2O-Hg-Hg-OH2]2+ center, with a Hg-Hg distance of 254 pm.

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.

Plutonium (IV) nitrate is an inorganic compound, a salt of plutonium and nitric acid with the chemical formula Pu(NO3)4. The compound dissolves in water and forms crystalline hydrates as dark green crystals.
Neptunium(IV) nitrate is an inorganic compound, a salt of neptunium and nitric acid with the chemical formula Np(NO3)4. The compound forms gray crystals, dissolves in water, and forms crystal hydrates.

Actinium(III) nitrate is an inorganic compound, actinium salt of nitric acid with the chemical formula Ac(NO3)3. The compound looks like white substance, readily soluble in water.
Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.

Holmium (III) nitrate is an inorganic compound, a salt of holmium and nitric acid with the chemical formula Ho(NO3)3. The compound forms yellowish crystals, dissolves in water, also forms crystalline hydrates.

Ytterbium(III) nitrate is an inorganic compound, a salt of ytterbium and nitric acid with the chemical formula Yb(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates.
Lutetium(III) nitrate is an inorganic compound, a salt of lutetium and nitric acid with the chemical formula Lu(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is poisonous.

Erbium(III) nitrate is an inorganic compound, a salt of erbium and nitric acid with the chemical formula Er(NO3)3. The compound forms pink crystals, readily soluble in water, also forms crystalline hydrates.
Curium(III) nitrate is an inorganic compound, a salt of curium and nitric acid with the chemical formula Cm(NO3)3.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.
Promethium(III) nitrate is an inorganic compound, a salt of promethium and nitric acid with the chemical formula Pm(NO3)3. The compound is radioactive, soluble in water and forms crystalline hydrates.
Polonium tetranitrate is an inorganic compound, a salt of polonium and nitric acid with the chemical formula Po(NO3)4. The compound is radioactive, forms white crystals.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.

















