
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, gallium is in group 13 of the periodic table and is similar to the other metals of the group.

Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium.

Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula MgSO4, consisting of magnesium cations Mg2+ (20.19% by mass) and sulfate anions SO2−4. It is a white crystalline solid, soluble in water but not in ethanol.

Magnesium is an essential element in biological systems. Magnesium occurs typically as the Mg2+ ion. It is an essential mineral nutrient (i.e., element) for life and is present in every cell type in every organism. For example, adenosine triphosphate (ATP), the main source of energy in cells, must bind to a magnesium ion in order to be biologically active. What is called ATP is often actually Mg-ATP. As such, magnesium plays a role in the stability of all polyphosphate compounds in the cells, including those associated with the synthesis of DNA and RNA.

Parathyroid hormone (PTH), also called parathormone or parathyrin, is a peptide hormone secreted by the parathyroid glands that regulates the serum calcium concentration through its effects on bone, kidney, and intestine.

Calcitonin is a 32 amino acid peptide hormone secreted by parafollicular cells (also known as C cells) of the thyroid (or endostyle) in humans and other chordates in the ultimopharyngeal body. It acts to reduce blood calcium (Ca2+), opposing the effects of parathyroid hormone (PTH).
Hypercalcemia, also spelled hypercalcaemia, is a high calcium (Ca2+) level in the blood serum. The normal range is 2.1–2.6 mmol/L (8.8–10.7 mg/dL, 4.3–5.2 mEq/L), with levels greater than 2.6 mmol/L defined as hypercalcemia. Those with a mild increase that has developed slowly typically have no symptoms. In those with greater levels or rapid onset, symptoms may include abdominal pain, bone pain, confusion, depression, weakness, kidney stones or an abnormal heart rhythm including cardiac arrest.

Hydroxycarbamide, also known as hydroxyurea, is a medication used in sickle-cell disease, essential thrombocythemia, chronic myelogenous leukemia, polycythemia vera, and cervical cancer. In sickle-cell disease it increases fetal hemoglobin and decreases the number of attacks. It is taken by mouth.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include: calcium, chloride, magnesium, phosphate, potassium, and sodium.
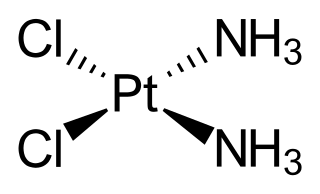
Cisplatin is a chemotherapy medication used to treat a number of cancers. These include testicular cancer, ovarian cancer, cervical cancer, bladder cancer, head and neck cancer, esophageal cancer, lung cancer, mesothelioma, brain tumors and neuroblastoma. It is given by injection into a vein.

Epirubicin is an anthracycline drug used for chemotherapy. It can be used in combination with other medications to treat breast cancer in patients who have had surgery to remove the tumor. It is marketed by Pfizer under the trade name Ellence in the US and Pharmorubicin or Epirubicin Ebewe elsewhere.

Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).
Non-small-cell lung cancer (NSCLC), or non-small-cell lung carcinoma, is any type of epithelial lung cancer other than small-cell lung cancer (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively and postoperatively.

Gallium maltolate is a coordination complex consisting of a trivalent gallium cation coordinated to three maltolate ligands. The compound is a potential therapeutic agent for cancer, infectious disease, and inflammatory disease. A cosmetic skin cream containing gallium maltolate is marketed under the name Gallixa. It is a colorless solid with significant solubility in both water and lipids.

Genta Incorporated was a biopharmaceutical company started in La Jolla, California, which discovered and developed innovative drugs for the treatment of patients with cancer. Founded in 1989 by a highly skilled entrepreneur, the company focused on a novel technology known as antisense, which targets gene products that are associated with the onset and progression of serious diseases. At that time, only Ionis Pharmaceuticals, Inc. was conducting significant research with this technology. Antisense is a short span of oligonucleotides – modified DNA structures ranging from about 12-24 bases that selectively bind to specific RNA. The intent is to block expression of an aberrant protein that contributes to the disease of interest. Genta in-licensed three different antisense molecules that blocked Bcl-2, a fibroblast growth factor (FGF), and the gene c-myb, respectively.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.
Metal transporter CNNM3 is a human transmembrane protein which is made up of 707 amino acids. Although CNNM3 is ubiquitous, it is mostly present in the kidney, brain, lung, spleen, heart and liver.
Gallium compounds are compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2. There are also compounds of gallium with negative oxidation states, ranging from -5 to -1, most of these compounds being magnesium gallides (MgxGay).


















