
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.
Methanotrophs are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to survive.
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, forming dinitrosyl iron complexes. In most Fe–S proteins, the terminal ligands on Fe are thiolate, but exceptions exist.

Methane monooxygenase (MMO) is an enzyme capable of oxidizing the C-H bond in methane as well as other alkanes. Methane monooxygenase belongs to the class of oxidoreductase enzymes.

Inositol oxygenase, also commonly referred to as myo-inositol oxygenase (MIOX), is a non-heme di-iron enzyme that oxidizes myo-inositol to glucuronic acid. The enzyme employs a unique four-electron transfer at its Fe(II)/Fe(III) coordination sites and the reaction proceeds through the direct binding of myo-inositol followed by attack of the iron center by diatomic oxygen. This enzyme is part of the only known pathway for the catabolism of inositol in humans and is expressed primarily in the kidneys. Recent medical research regarding MIOX has focused on understanding its role in metabolic and kidney diseases such as diabetes, obesity and acute kidney injury. Industrially-focused engineering efforts are centered on improving MIOX activity in order to produce glucaric acid in heterologous hosts.

In enzymology, a camphor 5-monooxygenase (EC 1.14.15.1) is an enzyme that catalyzes the chemical reaction
Enzyme mimic is a branch of biomimetic chemistry, which aims at imitating the function of natural enzymes. An enzyme mimic is a small molecule complex that models the molecular structure, spectroscopic properties, or reactivity of an enzyme, sometimes called bioinspired complexes.
Dioxygen complexes are coordination compounds that contain O2 as a ligand. The study of these compounds is inspired by oxygen-carrying proteins such as myoglobin, hemoglobin, hemerythrin, and hemocyanin. Several transition metals form complexes with O2, and many of these complexes form reversibly. The binding of O2 is the first step in many important phenomena, such as cellular respiration, corrosion, and industrial chemistry. The first synthetic oxygen complex was demonstrated in 1938 with cobalt(II) complex reversibly bound O2.

Stephen James Lippard is the Arthur Amos Noyes Emeritus Professor of Chemistry at the Massachusetts Institute of Technology. He is considered one of the founders of bioinorganic chemistry, studying the interactions of nonliving substances such as metals with biological systems. He is also considered a founder of metalloneurochemistry, the study of metal ions and their effects in the brain and nervous system. He has done pioneering work in understanding protein structure and synthesis, the enzymatic functions of methane monooxygenase (MMO), and the mechanisms of cisplatin anticancer drugs. His work has applications for the treatment of cancer, for bioremediation of the environment, and for the development of synthetic methanol-based fuels.
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligands stabilize high oxidation states of a metal. They are also found in several metalloproteins, for example in molybdenum cofactors and in many iron-containing enzymes. One of the earliest synthetic compounds to incorporate an oxo ligand is potassium ferrate (K2FeO4), which was likely prepared by Georg E. Stahl in 1702.
Methanobactin (mb) is a class of copper-binding and reducing chromophoric peptides initially identified in the methanotroph Methylococcus capsulatus Bath - and later in Methylosinus trichosporium OB3b - during the isolation of the membrane-associated or particulate methane monooxygenase (pMMO). It is thought to be secreted to the extracellular media to recruit copper, a critical component of methane monooxygenase, the first enzyme in the series that catalyzes the oxidation of methane into methanol. Methanobactin functions as a chalkophore, similar to iron siderophores, by binding to Cu(II) or Cu(I) then shuttling the copper into the cell. Methanobactin has an extremely high affinity for binding and Cu(I) with a Kd of approximately 1020 M−1 at pH 8. Additionally, methanobactin can reduce Cu(II), which is toxic to cells, to Cu(I), the form used in pMMO. Moreover, different species of methanobactin are hypothesized to be ubiquitous within the biosphere, especially in light of the discovery of molecules produced by other type II methanotrophs that similarly bind and reduce copper (II) to copper (I).

Methane monooxygenase (particulate) (EC 1.14.18.3) is an enzyme with systematic name methane,quinol:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction
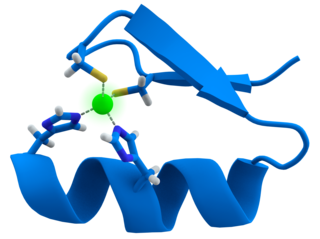
Transition metal thiolate complexes are metal complexes containing thiolate ligands. Thiolates are ligands that can be classified as soft Lewis bases. Therefore, thiolate ligands coordinate most strongly to metals that behave as soft Lewis acids as opposed to those that behave as hard Lewis acids. Most complexes contain other ligands in addition to thiolate, but many homoleptic complexes are known with only thiolate ligands. The amino acid cysteine has a thiol functional group, consequently many cofactors in proteins and enzymes feature cysteinate-metal cofactors.
Armando José Latourrette de Oliveira Pombeiro is a Portuguese chemical engineer.

Acetyl-CoA synthase (ACS), not to be confused with acetyl-CoA synthetase or acetate-CoA ligase, is a nickel-containing enzyme involved in the metabolic processes of cells. Together with carbon monoxide dehydrogenase (CODH), it forms the bifunctional enzyme Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) found in anaerobic organisms such as archaea and bacteria. The ACS/CODH enzyme works primarily through the Wood–Ljungdahl pathway which converts carbon dioxide to Acetyl-CoA. The recommended name for this enzyme is CO-methylating acetyl-CoA synthase.
Samaresh Mitra is an Indian bioinorganic chemist and an INSA Senior Scientist at the Indian Institute of Chemical Biology (IICB). He is known for his research on inorganic paramagnetic complexes and low-symmetry transition metal complexes. He is an elected fellow of the Indian National Science Academy, the National Academy of Sciences, India and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1983, for his contributions to chemical sciences.
Serena DeBeer is an American chemist. She is currently a W3-Professor and the director at the Max Planck Institute for Chemical Energy Conversion in Muelheim an der Ruhr, Germany, where she heads the Department of Inorganic Spectroscopy. Her expertise lies in the application and development of X-ray based spectroscopic methods as probes of electronic structure in biological and chemical catalysis.
Amy M. Barrios is an American medicinal chemist working as a professor of Medicinal Chemistry and the Associate Dean for Postdoctoral Affairs for the University of Utah. Barrios' research lab focuses on developing probes to study protein tyrosine phosphatase (PTP) activity and regulation.
Amie Kathleen Boal is an American chemist. She is an associate professor of chemistry, biochemistry, and molecular biology at Pennsylvania State University. In 2020, Boal was the recipient of the Pfizer Award in Enzyme Chemistry from the American Chemical Society.









