
Apoptosis is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day.

The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

Phagocytosis is the process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte.

Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae. Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery. Phagocytes occur in many species; some amoebae behave like macrophage phagocytes, which suggests that phagocytes appeared early in the evolution of life.

Protein S is a vitamin K-dependent plasma glycoprotein synthesized in the liver. In the circulation, Protein S exists in two forms: a free form and a complex form bound to complement protein C4b-binding protein (C4BP). In humans, protein S is encoded by the PROS1 gene. Protein S plays a role in coagulation.
Opsonins are extracellular proteins that, when bound to substances or cells, induce phagocytes to phagocytose the substances or cells with the opsonins bound. Thus, opsonins act as tags to label things in the body that should be phagocytosed by phagocytes. Different types of things ("targets") can be tagged by opsonins for phagocytosis, including: pathogens, cancer cells, aged cells, dead or dying cells, excess synapses, or protein aggregates. Opsonins help clear pathogens, as well as dead, dying and diseased cells.
Annexin is a common name for a group of cellular proteins. They are mostly found in eukaryotic organisms.
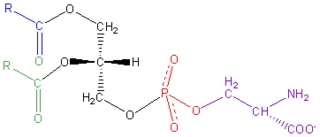
Phosphatidylserine is a phospholipid and is a component of the cell membrane. It plays a key role in cell cycle signaling, specifically in relation to apoptosis. It is a key pathway for viruses to enter cells via apoptotic mimicry. Its exposure on the outer surface of a membrane marks the cell for destruction via apoptosis.
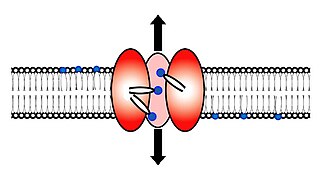
Scramblase is a protein responsible for the translocation of phospholipids between the two monolayers of a lipid bilayer of a cell membrane. In humans, phospholipid scramblases (PLSCRs) constitute a family of five homologous proteins that are named as hPLSCR1–hPLSCR5. Scramblases are not members of the general family of transmembrane lipid transporters known as flippases. Scramblases are distinct from flippases and floppases. Scramblases, flippases, and floppases are three different types of enzymatic groups of phospholipid transportation enzymes. The inner-leaflet, facing the inside of the cell, contains negatively charged amino-phospholipids and phosphatidylethanolamine. The outer-leaflet, facing the outside environment, contains phosphatidylcholine and sphingomyelin. Scramblase is an enzyme, present in the cell membrane, that can transport (scramble) the negatively charged phospholipids from the inner-leaflet to the outer-leaflet, and vice versa.

Flippases are transmembrane lipid transporter proteins located in the membrane which belong to ABC transporter or P4-type ATPase families. They are responsible for aiding the movement of phospholipid molecules between the two leaflets that compose a cell's membrane. The possibility of active maintenance of an asymmetric distribution of molecules in the phospholipid bilayer was predicted in the early 1970s by Mark Bretscher. Although phospholipids diffuse rapidly in the plane of the membrane, their polar head groups cannot pass easily through the hydrophobic center of the bilayer, limiting their diffusion in this dimension. Some flippases - often instead called scramblases - are energy-independent and bidirectional, causing reversible equilibration of phospholipid between the two sides of the membrane, whereas others are energy-dependent and unidirectional, using energy from ATP hydrolysis to pump the phospholipid in a preferred direction. Flippases are described as transporters that move lipids from the exoplasmic to the cytosolic face, while floppases transport in the reverse direction.

Annexin A1, also known as lipocortin I, is a protein that is encoded by the ANXA1 gene in humans.

Annexin A5 is a cellular protein in the annexin group. In flow cytometry, annexin V is commonly used to detect apoptotic cells by its ability to bind to phosphatidylserine, a marker of apoptosis when it is on the outer leaflet of the plasma membrane. The function of the protein is unknown; however, annexin A5 has been proposed to play a role in the inhibition of blood coagulation by competing for phosphatidylserine binding sites with prothrombin and also to inhibit the activity of phospholipase A1. These properties have been found by in vitro experiments.

Microvesicles are a type of extracellular vesicle (EV) that are released from the cell membrane. In multicellular organisms, microvesicles and other EVs are found both in tissues and in many types of body fluids. Delimited by a phospholipid bilayer, microvesicles can be as small as the smallest EVs or as large as 1000 nm. They are considered to be larger, on average, than intracellularly-generated EVs known as exosomes. Microvesicles play a role in intercellular communication and can transport molecules such as mRNA, miRNA, and proteins between cells.

Annexin A8-like protein 2 is a protein that in humans is encoded by the ANXA8L2 gene.
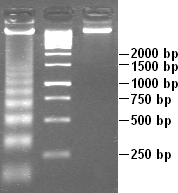
Apoptotic DNA fragmentation is a key feature of apoptosis, a type of programmed cell death. Apoptosis is characterized by the activation of endogenous endonucleases, particularly the caspase-3 activated DNase (CAD), with subsequent cleavage of nuclear DNA into internucleosomal fragments of roughly 180 base pairs (bp) and multiples thereof (360, 540 etc.). The apoptotic DNA fragmentation is being used as a marker of apoptosis and for identification of apoptotic cells either via the DNA laddering assay, the TUNEL assay, or the by detection of cells with fractional DNA content ("sub G1 cells") on DNA content frequency histograms e.g. as in the Nicoletti assay.

Anti-double stranded DNA (Anti-dsDNA) antibodies are a group of anti-nuclear antibodies (ANA) the target antigen of which is double stranded DNA. Blood tests such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-dsDNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis.
Apoptotic-cell associated molecular patterns (ACAMPs) are molecular markers present on cells which are going through apoptosis, i.e. programmed cell death. The term was used for the first time by C. D. Gregory in 2000. Recognition of these patterns by the pattern recognition receptors (PRRs) of phagocytes then leads to phagocytosis of the apoptotic cell. These patterns include eat-me signals on the apoptotic cells, loss of don’t-eat-me signals on viable cells and come-get-me signals ) secreted by the apoptotic cells in order to attract phagocytes. Thanks to these markers, apoptotic cells, unlike necrotic cells, do not trigger the unwanted immune response.

Regulator of microtubule dynamics protein 3 (RMDN3), more commonly known as Protein tyrosine phosphatase interacting protein 51 (PTPIP51), is a protein that in humans is encoded by the RMDN3 gene on chromosome 15. This protein contributes to multiple biological functions, including cellular differentiation, proliferation, motility, cytoskeleton formation, and apoptosis, and has been associated with numerous cancers.
The KX Blood-group Antigen (KXA) Family (TC# 2.A.112) consists of transport proteins that are part of the TOG superfamily. The KX gene codes for a novel protein with characteristics of membrane transporters that has been proposed to be a Na+ -dependent neutral amine and/or oligopeptide transporter. It is predicted to be 444 amino acyl residues in length and exhibits 10 putative transmembrane α-helical segments. The KX blood group antigen mRNA expression pattern correlates with McLeod syndrome.

Eat-me signals are molecules exposed on the surface of a cell to induce phagocytes to phagocytose (eat) that cell. Currently known eat-me signals include: phosphatidylserine, oxidized phospholipids, sugar residues, deoxyribonucleic acid (DNA), calreticulin, annexin A1, histones and pentraxin-3 (PTX3).
















