
The neutron is a subatomic particle, symbol
n
or
n0
, which has a neutral charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one dalton, they are both referred to as nucleons. Their properties and interactions are described by nuclear physics. Protons and neutrons are not elementary particles; each is composed of three quarks.

A neutrino is a fermion that interacts only via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small (-ino) that it was long thought to be zero. The rest mass of the neutrino is much smaller than that of the other known elementary particles. The weak force has a very short range, the gravitational interaction is extremely weak due to the very small mass of the neutrino, and neutrinos do not participate in the electromagnetic interaction or the strong interaction. Thus, neutrinos typically pass through normal matter unimpeded and undetected.
Neutronium is a hypothetical substance composed purely of neutrons. The word was coined by scientist Andreas von Antropoff in 1926 for the hypothetical "element of atomic number zero" that he placed at the head of the periodic table. However, the meaning of the term has changed over time, and from the last half of the 20th century onward it has been also used to refer to extremely dense substances resembling the neutron-degenerate matter theorized to exist in the cores of neutron stars; hereinafter "degenerate neutronium" will refer to this.

In nuclear physics, the island of stability is a predicted set of isotopes of superheavy elements that may have considerably longer half-lives than known isotopes of these elements. It is predicted to appear as an "island" in the chart of nuclides, separated from known stable and long-lived primordial radionuclides. Its theoretical existence is attributed to stabilizing effects of predicted "magic numbers" of protons and neutrons in the superheavy mass region.

The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total distance travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travelling through a small sphere of radius in a time interval, divided by a maximal cross section of the sphere and by the duration of the time interval. The dimension of neutron flux is and the usual unit is cm−2s−1.
Thorium (90Th) has seven naturally occurring isotopes but none are stable. One isotope, 232Th, is relatively stable, with a half-life of 1.405×1010 years, considerably longer than the age of the Earth, and even slightly longer than the generally accepted age of the universe. This isotope makes up nearly all natural thorium, so thorium was considered to be mononuclidic. However, in 2013, IUPAC reclassified thorium as binuclidic, due to large amounts of 230Th in deep seawater. Thorium has a characteristic terrestrial isotopic composition and thus a standard atomic weight can be given.
Naturally occurring xenon (54Xe) consists of seven stable isotopes and two very long-lived isotopes. Double electron capture has been observed in 124Xe and double beta decay in 136Xe, which are among the longest measured half-lives of all nuclides. The isotopes 126Xe and 134Xe are also predicted to undergo double beta decay, but this has never been observed in these isotopes, so they are considered to be stable. Beyond these stable forms, 32 artificial unstable isotopes and various isomers have been studied, the longest-lived of which is 127Xe with a half-life of 36.345 days. All other isotopes have half-lives less than 12 days, most less than 20 hours. The shortest-lived isotope, 108Xe, has a half-life of 58 μs, and is the heaviest known nuclide with equal numbers of protons and neutrons. Of known isomers, the longest-lived is 131mXe with a half-life of 11.934 days. 129Xe is produced by beta decay of 129I ; 131mXe, 133Xe, 133mXe, and 135Xe are some of the fission products of both 235U and 239Pu, so are used as indicators of nuclear explosions.
Calcium (20Ca) has 26 known isotopes, ranging from 35Ca to 60Ca. There are five stable isotopes, plus one isotope (48Ca) with such a long half-life that for all practical purposes it can be considered stable. The most abundant isotope, 40Ca, as well as the rare 46Ca, are theoretically unstable on energetic grounds, but their decay has not been observed. Calcium also has a cosmogenic isotope, radioactive 41Ca, which has a half-life of 99,400 years. Unlike cosmogenic isotopes that are produced in the atmosphere, 41Ca is produced by neutron activation of 40Ca. Most of its production is in the upper metre of the soil column, where the cosmogenic neutron flux is still sufficiently strong. 41Ca has received much attention in stellar studies because it decays to 41K, a critical indicator of solar system anomalies. The most stable artificial radioisotopes are 45Ca with a half-life of 163 days and 47Ca with a half-life of 4.5 days. All other calcium isotopes have half-lives measured in minutes or less.
Chlorine (17Cl) has 25 isotopes, ranging from 28Cl to 52Cl, and two isomers, 34mCl and 38mCl. There are two stable isotopes, 35Cl (75.77%) and 37Cl (24.23%), giving chlorine a standard atomic weight of 35.45. The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years. All other isotopes have half-lives under 1 hour, many less than one second. The shortest-lived are proton-unbound 29Cl and 30Cl, with half-lives less than 10 picoseconds and 30 nanoseconds, respectively; the half-life of 28Cl is unknown.
Flerovium (114Fl) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 289Fl in 1999. Flerovium has six known isotopes, along with the unconfirmed 290Fl, and possibly two nuclear isomers. The longest-lived isotope is 289Fl with a half-life of 1.9 seconds, but 290Fl may have a longer half-life of 19 seconds.
Bismuth-209 (209Bi) is the isotope of bismuth with the longest known half-life of any radioisotope that undergoes α-decay. It has 83 protons and a magic number of 126 neutrons, and an atomic mass of 208.9803987 amu. Primordial bismuth consists entirely of this isotope.

The Daya Bay Reactor Neutrino Experiment is a China-based multinational particle physics project studying neutrinos, in particular neutrino oscillations. The multinational collaboration includes researchers from China, Chile, the United States, Taiwan, Russia, and the Czech Republic. The US side of the project is funded by the US Department of Energy's Office of High Energy Physics.
G. Norris Glasoe was an American nuclear physicist. He was a member of the Columbia University team which was the first in the United States to verify the European discovery of the nuclear fission of uranium via neutron bombardment. During World War II, he worked at the MIT Radiation Laboratory. He was a physicist and administrator at the Brookhaven National Laboratory.
The Modular Neutron Array (MoNA) is a large-area, high-efficiency neutron detector that is used in basic research of rare isotopes at Michigan State University's National Superconducting Cyclotron Laboratory (NSCL), a nuclear physics research facility. It is specifically designed for detecting neutrons stemming from breakup reactions of fast fragmentation beams.
Sherry J. Yennello is an American nuclear chemist and an Elected Fellow of the American Association for the Advancement of Science. She is a Regents Professor and the holder of the Cyclotron Institute Bright Chair in Nuclear Science, who currently serves as the Director of the Cyclotron Institute at Texas A&M University. She is also a Fellow of the American Chemical Society and the American Physical Society. She has authored as well as co-authored more than 530 peer reviewed journal articles and has conducted many invited talks, presentations and seminars at several prestigious academic conferences and scholarly lectures.
Virginia Ruth Brown was an American nuclear physicist known for her contributions on the structure, interaction, reactions, and bremsstrahlung of nucleons and atomic nuclei. She spent most of her career as a researcher at the Lawrence Livermore National Laboratory.

Volker D. Burkert is a German physicist, academic and researcher. He is a Principal Staff Scientist at the Thomas Jefferson National Accelerator Facility at Jefferson Lab (JLab) in Newport News, Virginia (USA). He has made major contributions to the design of the CEBAF Large Acceptance Spectrometer (CLAS) that made it suitable for high luminosity operation in experiments studying spin-polarized electron scattering.

Michael C. F. Wiescher is a German-American experimental nuclear physicist and astrophysicist, known for his laboratory research in nuclear physics connected with various astrophysical phenomena such as stellar evolution and explosion environments.
Daniel S. Akerib is an American particle physicist and astrophysicist. He was elected in 2008 a fellow of the American Physical Society (APS).
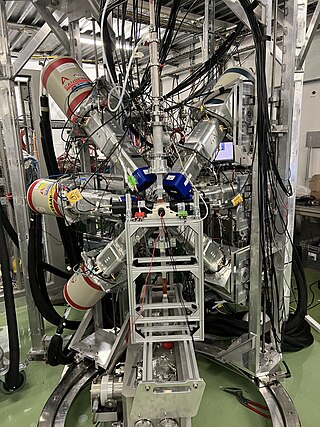
The ISOLDE Decay Station (IDS) is a permanent experiment located in the ISOLDE facility at CERN. The purpose of the experiment is to measure decay properties of radioactive isotopes using spectroscopy techniques for a variety of applications, including nuclear engineering and astrophysics. The experimental setup has been operational since 2014.







