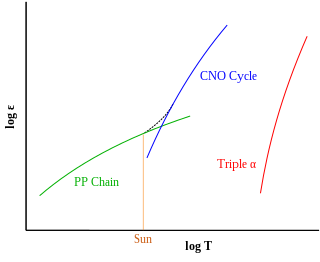
The CNO cycle is one of the two known sets of fusion reactions by which stars convert hydrogen to helium, the other being the proton–proton chain reaction, which is more efficient at the Sun's core temperature. The CNO cycle is hypothesized to be dominant in stars that are more than 1.3 times as massive as the Sun.

The proton–proton chain, also commonly referred to as the p–p chain, is one of two known sets of nuclear fusion reactions by which stars convert hydrogen to helium. It dominates in stars with masses less than or equal to that of the Sun, whereas the CNO cycle, the other known reaction, is suggested by theoretical models to dominate in stars with masses greater than about 1.3 times that of the Sun.

Stellar evolution is the process by which a star changes over the course of time. Depending on the mass of the star, its lifetime can range from a few million years for the most massive to trillions of years for the least massive, which is considerably longer than the current age of the universe. The table shows the lifetimes of stars as a function of their masses. All stars are formed from collapsing clouds of gas and dust, often called nebulae or molecular clouds. Over the course of millions of years, these protostars settle down into a state of equilibrium, becoming what is known as a main-sequence star.
In physical cosmology, Big Bang nucleosynthesis is the production of nuclei other than those of the lightest isotope of hydrogen during the early phases of the universe. This type of nucleosynthesis is thought by most cosmologists to have occurred from 10 seconds to 20 minutes after the Big Bang. It is thought to be responsible for the formation of most of the universe's helium, along with small fractions of the hydrogen isotope deuterium, the helium isotope helium-3 (3He), and a very small fraction of the lithium isotope lithium-7 (7Li). In addition to these stable nuclei, two unstable or radioactive isotopes were produced: the heavy hydrogen isotope tritium and the beryllium isotope beryllium-7 (7Be). These unstable isotopes later decayed into 3He and 7Li, respectively, as above.
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons and nuclei. According to current theories, the first nuclei were formed a few minutes after the Big Bang, through nuclear reactions in a process called Big Bang nucleosynthesis. After about 20 minutes, the universe had expanded and cooled to a point at which these high-energy collisions among nucleons ended, so only the fastest and simplest reactions occurred, leaving our universe containing hydrogen and helium. The rest is traces of other elements such as lithium and the hydrogen isotope deuterium. Nucleosynthesis in stars and their explosions later produced the variety of elements and isotopes that we have today, in a process called cosmic chemical evolution. The amounts of total mass in elements heavier than hydrogen and helium remains small, so that the universe still has approximately the same composition.

Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a predictive theory, it yields accurate estimates of the observed abundances of the elements. It explains why the observed abundances of elements change over time and why some elements and their isotopes are much more abundant than others. The theory was initially proposed by Fred Hoyle in 1946, who later refined it in 1954. Further advances were made, especially to nucleosynthesis by neutron capture of the elements heavier than iron, by Margaret and Geoffrey Burbidge, William Alfred Fowler and Fred Hoyle in their famous 1957 B2FH paper, which became one of the most heavily cited papers in astrophysics history.

The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements. The other class is a cycle of reactions called the triple-alpha process, which consumes only helium, and produces carbon. The alpha process most commonly occurs in massive stars and during supernovae.
The carbon-burning process or carbon fusion is a set of nuclear fusion reactions that take place in the cores of massive stars (at least 8 at birth) that combines carbon into other elements. It requires high temperatures (> 5×108 K or 50 keV) and densities (> 3×109 kg/m3).
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main sequence on the Hertzsprung–Russell diagram. It follows the previous stages of hydrogen, helium, carbon, neon and oxygen burning processes.

In nuclear astrophysics, the rapid neutron-capture process, also known as the r-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", with the other half produced by the p-process and s-process. The r-process usually synthesizes the most neutron-rich stable isotopes of each heavy element. The r-process can typically synthesize the heaviest four isotopes of every heavy element, and the two heaviest isotopes, which are referred to as r-only nuclei, can be created via the r-process only. Abundance peaks for the r-process occur near mass numbers A = 82, A = 130 and A = 196.
The slow neutron-capture process, or s-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The s-process is responsible for the creation (nucleosynthesis) of approximately half the atomic nuclei heavier than iron.
The term p-process (p for proton) is used in two ways in the scientific literature concerning the astrophysical origin of the elements (nucleosynthesis). Originally it referred to a proton capture process which is the source of certain, naturally occurring, neutron-deficient isotopes of the elements from selenium to mercury. These nuclides are called p-nuclei and their origin is still not completely understood. Although it was shown that the originally suggested process cannot produce the p-nuclei, later on the term p-process was sometimes used to generally refer to any nucleosynthesis process supposed to be responsible for the p-nuclei.
Supernova nucleosynthesis is the nucleosynthesis of chemical elements in supernova explosions.
In physical cosmology, the Alpher–Bethe–Gamow paper, or αβγ paper, was created by Ralph Alpher, then a physics PhD student, his advisor George Gamow, and Hans Bethe. The work, which would become the subject of Alpher's PhD dissertation, argued that the Big Bang would create hydrogen, helium and heavier elements in the correct proportions to explain their abundance in the early universe. While the original theory neglected a number of processes important to the formation of heavy elements, subsequent developments showed that Big Bang nucleosynthesis is consistent with the observed constraints on all primordial elements.

A Type II supernova or SNII results from the rapid collapse and violent explosion of a massive star. A star must have at least eight times, but no more than 40 to 50 times, the mass of the Sun (M☉) to undergo this type of explosion. Type II supernovae are distinguished from other types of supernovae by the presence of hydrogen in their spectra. They are usually observed in the spiral arms of galaxies and in H II regions, but not in elliptical galaxies; those are generally composed of older, low-mass stars, with few of the young, very massive stars necessary to cause a supernova.
The B2FH paper was a landmark scientific paper on the origin of the chemical elements. The paper's title is Synthesis of the Elements in Stars, but it became known as B2FH from the initials of its authors: Margaret Burbidge, Geoffrey Burbidge, William A. Fowler, and Fred Hoyle. It was written from 1955 to 1956 at the University of Cambridge and Caltech, then published in Reviews of Modern Physics in 1957.
The Hans A. Bethe Prize, is presented annually by the American Physical Society. The prize honors outstanding work in theory, experiment or observation in the areas of astrophysics, nuclear physics, nuclear astrophysics, or closely related fields. The prize consists of $10,000 and a certificate citing the contributions made by the recipient.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
p-nuclei (p stands for proton-rich) are certain proton-rich, naturally occurring isotopes of some elements between selenium and mercury inclusive which cannot be produced in either the s- or the r-process.

Donald Delbert Clayton is an American astrophysicist whose most visible achievement was the prediction from nucleosynthesis theory that supernovae are intensely radioactive. That earned Clayton the NASA Exceptional Scientific Achievement Medal (1992) for “theoretical astrophysics related to the formation of (chemical) elements in the explosions of stars and to the observable products of these explosions”. Supernovae thereafter became the most important stellar events in astronomy owing to their profoundly radioactive nature. Not only did Clayton discover radioactive nucleosynthesis during explosive silicon burning in stars but he also predicted a new type of astronomy based on it, namely the associated gamma-ray line radiation emitted by matter ejected from supernovae. That paper was selected as one of the fifty most influential papers in astronomy during the twentieth century for the Centennial Volume of the American Astronomical Society. He gathered support from influential astronomers and physicists for a new NASA budget item for a gamma-ray-observatory satellite, achieving successful funding for Compton Gamma Ray Observatory. With his focus on radioactive supernova gas Clayton discovered a new chemical pathway causing carbon dust to condense there by a process that is activated by the radioactivity.








