Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).

Collagen is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis, and Vitamin E improves the production of collagen.

A cell wall is a structural layer that surrounds some cell types, found immediately outside the cell membrane. It can be tough, flexible, and sometimes rigid. Primarily, it provides the cell with structural support, shape, protection, and functions as a selective barrier. Another vital role of the cell wall is to help the cell withstand osmotic pressure and mechanical stress. While absent in many eukaryotes, including animals, cell walls are prevalent in other organisms such as fungi, algae and plants, and are commonly found in most prokaryotes, with the exception of mollicute bacteria.

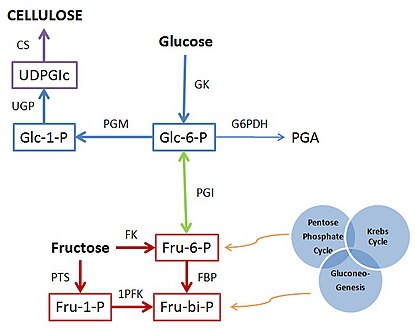
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.
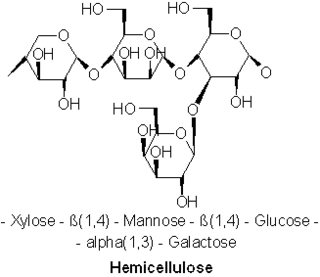
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Polysaccharides, or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin.
Acetic acid bacteria (AAB) are a group of Gram-negative bacteria which oxidize sugars or ethanol and produce acetic acid during fermentation. The acetic acid bacteria consist of 10 genera in the family Acetobacteraceae. Several species of acetic acid bacteria are used in industry for production of certain foods and chemicals.

An exoenzyme, or extracellular enzyme, is an enzyme that is secreted by a cell and functions outside that cell. Exoenzymes are produced by both prokaryotic and eukaryotic cells and have been shown to be a crucial component of many biological processes. Most often these enzymes are involved in the breakdown of larger macromolecules. The breakdown of these larger macromolecules is critical for allowing their constituents to pass through the cell membrane and enter into the cell. For humans and other complex organisms, this process is best characterized by the digestive system which breaks down solid food via exoenzymes. The small molecules, generated by the exoenzyme activity, enter into cells and are utilized for various cellular functions. Bacteria and fungi also produce exoenzymes to digest nutrients in their environment, and these organisms can be used to conduct laboratory assays to identify the presence and function of such exoenzymes. Some pathogenic species also use exoenzymes as virulence factors to assist in the spread of these disease-causing microorganisms. In addition to the integral roles in biological systems, different classes of microbial exoenzymes have been used by humans since pre-historic times for such diverse purposes as food production, biofuels, textile production and in the paper industry. Another important role that microbial exoenzymes serve is in the natural ecology and bioremediation of terrestrial and marine environments.

Polyhydroxyalkanoates or PHAs are polyesters produced in nature by numerous microorganisms, including through bacterial fermentation of sugars or lipids. When produced by bacteria they serve as both a source of energy and as a carbon store. More than 150 different monomers can be combined within this family to give materials with extremely different properties. These plastics are biodegradable and are used in the production of bioplastics.

Fibrils are structural biological materials found in nearly all living organisms. Not to be confused with fibers or filaments, fibrils tend to have diameters ranging from 10–100 nanometers. Fibrils are not usually found alone but rather are parts of greater hierarchical structures commonly found in biological systems. Due to the prevalence of fibrils in biological systems, their study is of great importance in the fields of microbiology, biomechanics, and materials science.

Natural fibers or natural fibres are fibers that are produced by geological processes, or from the bodies of plants or animals. They can be used as a component of composite materials, where the orientation of fibers impacts the properties. Natural fibers can also be matted into sheets to make paper or felt.

Symbiotic culture of bacteria and yeast (SCOBY) is a culinary symbiotic fermentation culture (starter) consisting of lactic acid bacteria (LAB), acetic acid bacteria (AAB), and yeast which arises in the preparation of sour foods and beverages such as kombucha. Beer and wine also undergo fermentation with yeast, but the lactic acid bacteria and acetic acid bacteria components unique to SCOBY are usually viewed as a source of spoilage rather than a desired addition. Both LAB and AAB enter on the surface of barley and malt in beer fermentation and grapes in wine fermentation; LAB lowers the pH of the beer/wine while AAB takes the ethanol produced from the yeast and oxidizes it further into vinegar, resulting in a sour taste and smell. AAB are also responsible for the formation of the cellulose SCOBY.

The UDP-forming form of cellulose synthase is the main enzyme that produces cellulose. Systematically, it is known as UDP-glucose:(1→4)-β-D-glucan 4-β-D-glucosyltransferase in enzymology. It catalyzes the chemical reaction:

Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.

Cyclic di-GMP is a second messenger used in signal transduction in a wide variety of bacteria. Cyclic di-GMP is not known to be used by archaea, and has only been observed in eukaryotes in Dictyostelium. The biological role of cyclic di-GMP was first uncovered when it was identified as an allosteric activator of a cellulose synthase found in Gluconacetobacter xylinus in order to produce microbial cellulose.

Cellulose fibers are fibers made with ethers or esters of cellulose, which can be obtained from the bark, wood or leaves of plants, or from other plant-based material. In addition to cellulose, the fibers may also contain hemicellulose and lignin, with different percentages of these components altering the mechanical properties of the fibers.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.

Nanocellulose is a term referring to nano-structured cellulose. This may be either cellulose nanocrystal, cellulose nanofibers (CNF) also called nanofibrillated cellulose (NFC), or bacterial nanocellulose, which refers to nano-structured cellulose produced by bacteria.

Acetobacter aceti is a Gram-negative bacterium that moves using its peritrichous flagella. Louis Pasteur proved it to be the cause of conversion of ethanol to acetic acid in 1864. It is a benign microorganism which is present everywhere in the environment, existing in alcoholic ecological niches which include flowers, fruits, honey bees, water and soil. This microbe lives wherever sugar fermentation occurs. It typically grows on substrates rich in sugars, like glucose or other carbon sources. It thrives best in temperatures that range from 25 to 30 degrees Celsius with a max temperature of 35 degrees Celsius and in pH that ranges from 5.5 to 6.3. For a long time it has been used in the fermentation industry to produce acetic acid from alcohol. A. aceti is an obligate aerobe, which means that it requires oxygen to grow as oxygen is used as the terminal electron acceptor.
Komagataeibacter xylinus is a species of bacteria best known for its ability to produce cellulose, specifically bacterial cellulose.