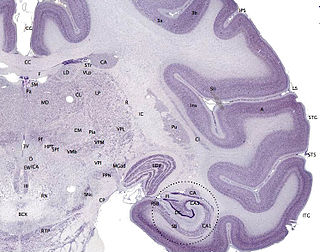
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. The cerebral cortex mostly consists of the six-layered neocortex, with just 10% consisting of the allocortex. It is separated into two cortices, by the longitudinal fissure that divides the cerebrum into the left and right cerebral hemispheres. The two hemispheres are joined beneath the cortex by the corpus callosum. The cerebral cortex is the largest site of neural integration in the central nervous system. It plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is part of the brain responsible for cognition.

Vibrissae, more generally called whiskers, are a type of stiff, functional hair used by mammals to sense their environment. These hairs are finely specialised for this purpose, whereas other types of hair are coarser as tactile sensors. Although whiskers are specifically those found around the face, vibrissae are known to grow in clusters at various places around the body. Most mammals have them, including all non-human primates and especially nocturnal mammals.
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.
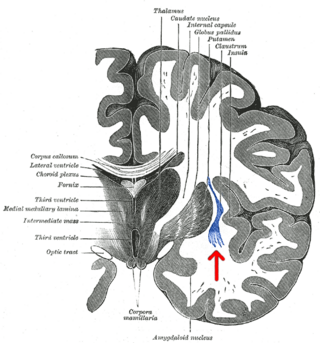
The claustrum is a thin sheet of neurons and supporting glial cells, that connects to the cerebral cortex and subcortical regions including the amygdala, hippocampus and thalamus of the brain. It is located between the insular cortex laterally and the putamen medially, encased by the extreme and external capsules respectively. Blood to the claustrum is supplied by the middle cerebral artery. It is considered to be the most densely connected structure in the brain, and thus hypothesized to allow for the integration of various cortical inputs such as vision, sound and touch, into one experience. Other hypotheses suggest that the claustrum plays a role in salience processing, to direct attention towards the most behaviorally relevant stimuli amongst the background noise. The claustrum is difficult to study given the limited number of individuals with claustral lesions and the poor resolution of neuroimaging.
Cortical maps are collections (areas) of minicolumns in the brain cortex that have been identified as performing a specific information processing function.
Multisensory integration, also known as multimodal integration, is the study of how information from the different sensory modalities may be integrated by the nervous system. A coherent representation of objects combining modalities enables animals to have meaningful perceptual experiences. Indeed, multisensory integration is central to adaptive behavior because it allows animals to perceive a world of coherent perceptual entities. Multisensory integration also deals with how different sensory modalities interact with one another and alter each other's processing.
Neuroplasticity, also known as neural plasticity, or brain plasticity, is the ability of neural networks in the brain to change through growth and reorganization. It is when the brain is rewired to function in some way that differs from how it previously functioned. These changes range from individual neuron pathways making new connections, to systematic adjustments like cortical remapping or neural oscillation. Other forms of neuroplasticity include homologous area adaptation, cross modal reassignment, map expansion, and compensatory masquerade. Examples of neuroplasticity include circuit and network changes that result from learning a new ability, information acquisition, environmental influences, practice, and psychological stress.

In neuroanatomy, thalamocortical radiations also known as thalamocortical fibres, are the efferent fibres that project from the thalamus to distinct areas of the cerebral cortex. They form fibre bundles that emerge from the lateral surface of the thalamus.

Stellate cells are neurons in the central nervous system, named for their star-like shape formed by dendritic processes radiating from the cell body. Many stellate cells are GABAergic and are located in the molecular layer of the cerebellum. Stellate cells are derived from dividing progenitor cells in the white matter of postnatal cerebellum. Dendritic trees can vary between neurons. There are two types of dendritic trees in the cerebral cortex, which include pyramidal cells, which are pyramid shaped and stellate cells which are star shaped. Dendrites can also aid neuron classification. Dendrites with spines are classified as spiny, those without spines are classified as aspinous. Stellate cells can be spiny or aspinous, while pyramidal cells are always spiny. Most common stellate cells are the inhibitory interneurons found within the upper half of the molecular layer in the cerebellum. Cerebellar stellate cells synapse onto the dendritic trees of Purkinje cells and send inhibitory signals. Stellate neurons are sometimes found in other locations in the central nervous system; cortical spiny stellate cells are found in layer IVC of the primary visual cortex. In the somatosensory barrel cortex of mice and rats, glutamatergic (excitatory) spiny stellate cells are organized in the barrels of layer 4. They receive excitatory synaptic fibres from the thalamus and process feed forward excitation to 2/3 layer of the primary visual cortex to pyramidal cells. Cortical spiny stellate cells have a 'regular' firing pattern. Stellate cells are chromophobes, that is cells that does not stain readily, and thus appears relatively pale under the microscope.
The zona incerta (ZI) is a horizontally elongated region of gray matter in the subthalamus below the thalamus. Its connections project extensively over the brain from the cerebral cortex down into the spinal cord.
A topographic map is the ordered projection of a sensory surface, like the retina or the skin, or an effector system, like the musculature, to one or more structures of the central nervous system. Topographic maps can be found in all sensory systems and in many motor systems.
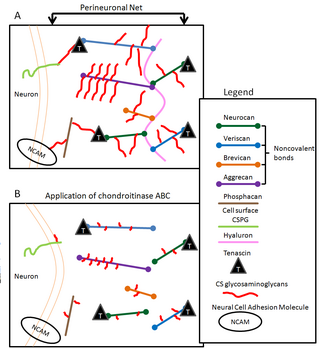
Perineuronal nets (PNNs) are specialized extracellular matrix structures responsible for synaptic stabilization in the adult brain. PNNs are found around certain neuron cell bodies and proximal neurites in the central nervous system. PNNs play a critical role in the closure of the childhood critical period, and their digestion can cause restored critical period-like synaptic plasticity in the adult brain. They are largely negatively charged and composed of chondroitin sulfate proteoglycans, molecules that play a key role in development and plasticity during postnatal development and in the adult.
Activity-dependent plasticity is a form of functional and structural neuroplasticity that arises from the use of cognitive functions and personal experience; hence, it is the biological basis for learning and the formation of new memories. Activity-dependent plasticity is a form of neuroplasticity that arises from intrinsic or endogenous activity, as opposed to forms of neuroplasticity that arise from extrinsic or exogenous factors, such as electrical brain stimulation- or drug-induced neuroplasticity. The brain's ability to remodel itself forms the basis of the brain's capacity to retain memories, improve motor function, and enhance comprehension and speech amongst other things. It is this trait to retain and form memories that is associated with neural plasticity and therefore many of the functions individuals perform on a daily basis. This plasticity occurs as a result of changes in gene expression which are triggered by signaling cascades that are activated by various signaling molecules during increased neuronal activity.

In physiology, the somatosensory system is the network of neural structures in the brain and body that produce the perception of touch, as well as temperature (thermoception), body position (proprioception), and pain. It is a subset of the sensory nervous system, which also represents visual, auditory, olfactory, gustatory and vestibular stimuli.

Cross modal plasticity is the adaptive reorganization of neurons to integrate the function of two or more sensory systems. Cross modal plasticity is a type of neuroplasticity and often occurs after sensory deprivation due to disease or brain damage. The reorganization of the neural network is greatest following long-term sensory deprivation, such as congenital blindness or pre-lingual deafness. In these instances, cross modal plasticity can strengthen other sensory systems to compensate for the lack of vision or hearing. This strengthening is due to new connections that are formed to brain cortices that no longer receive sensory input.

In animal physiology, hydrodynamic reception refers to the ability of some animals to sense water movements generated by biotic or abiotic sources. This form of mechanoreception is useful for orientation, hunting, predator avoidance, and schooling. Frequent encounters with conditions of low visibility can prevent vision from being a reliable information source for navigation and sensing objects or organisms in the environment. Sensing water movements is one resolution to this problem.
Sensory maps and brain development is a concept in neuroethology that links the development of the brain over an animal’s lifetime with the fact that there is spatial organization and pattern to an animal’s sensory processing. Sensory maps are the representations of sense organs as organized maps in the brain, and it is the fundamental organization of processing. Sensory maps are not always close to an exact topographic projection of the senses. The fact that the brain is organized into sensory maps has wide implications for processing, such as that lateral inhibition and coding for space are byproducts of mapping. The developmental process of an organism guides sensory map formation; the details are yet unknown. The development of sensory maps requires learning, long term potentiation, experience-dependent plasticity, and innate characteristics. There is significant evidence for experience-dependent development and maintenance of sensory maps, and there is growing evidence on the molecular basis, synaptic basis and computational basis of experience-dependent development.
Cortical remapping, also referred to as cortical reorganization, is the process by which an existing cortical map is affected by a stimulus resulting in the creating of a 'new' cortical map. Every part of the body is connected to a corresponding area in the brain which creates a cortical map. When something happens to disrupt the cortical maps such as an amputation or a change in neuronal characteristics, the map is no longer relevant. The part of the brain that is in charge of the amputated limb or neuronal change will be dominated by adjacent cortical regions that are still receiving input, thus creating a remapped area. Remapping can occur in the sensory or motor system. The mechanism for each system may be quite different. Cortical remapping in the somatosensory system happens when there has been a decrease in sensory input to the brain due to deafferentation or amputation, as well as a sensory input increase to an area of the brain. Motor system remapping receives more limited feedback that can be difficult to interpret.
Jessica Cardin is an American neuroscientist who is an associate professor of neuroscience at Yale University School of Medicine. Cardin's lab studies local circuits within the primary visual cortex to understand how cellular and synaptic interactions flexibly adapt to different behavioral states and contexts to give rise to visual perceptions and drive motivated behaviors. Cardin's lab applies their knowledge of adaptive cortical circuit regulation to probe how circuit dysfunction manifests in disease models.
Corey C. Harwell is an American neuroscientist who is an assistant professor in the Department of Neurobiology at Harvard Medical School.












