Tuberculosis (TB), also known as the "white death", or historically as consumption, is an infectious disease usually caused by Mycobacterium tuberculosis (MTB) bacteria. Tuberculosis generally affects the lungs, but it can also affect other parts of the body. Most infections show no symptoms, in which case it is known as latent tuberculosis. Around 10% of latent infections progress to active disease which, if left untreated, kill about half of those affected. Typical symptoms of active TB are chronic cough with blood-containing mucus, fever, night sweats, and weight loss. Infection of other organs can cause a wide range of symptoms.

Rifampicin, also known as rifampin, is an ansamycin antibiotic used to treat several types of bacterial infections, including tuberculosis (TB), Mycobacterium avium complex, leprosy, and Legionnaires' disease. It is almost always used together with other antibiotics with two notable exceptions: when given as a "preferred treatment that is strongly recommended" for latent TB infection; and when used as post-exposure prophylaxis to prevent Haemophilus influenzae type b and meningococcal disease in people who have been exposed to those bacteria. Before treating a person for a long period of time, measurements of liver enzymes and blood counts are recommended. Rifampicin may be given either by mouth or intravenously.

Tuberculosis management describes the techniques and procedures utilized for treating tuberculosis (TB).

Tibotec was a pharmaceutical company with a focus on research and development for the treatment of infectious diseases such as HIV/AIDS and hepatitis C. The company was founded in 1994 and then acquired by Johnson & Johnson and merged into its Janssen Pharmaceuticals division in 2002.

4-Aminosalicylic acid, also known as para-aminosalicylic acid (PAS) and sold under the brand name Paser among others, is an antibiotic primarily used to treat tuberculosis. Specifically it is used to treat active drug resistant tuberculosis together with other antituberculosis medications. It has also been used as a second line agent to sulfasalazine in people with inflammatory bowel disease such as ulcerative colitis and Crohn's disease. It is typically taken by mouth.

Amikacin is an antibiotic medication used for a number of bacterial infections. This includes joint infections, intra-abdominal infections, meningitis, pneumonia, sepsis, and urinary tract infections. It is also used for the treatment of multidrug-resistant tuberculosis. It is used by injection into a vein using an IV or into a muscle.

Moxifloxacin is an antibiotic, used to treat bacterial infections, including pneumonia, conjunctivitis, endocarditis, tuberculosis, and sinusitis. It can be given by mouth, by injection into a vein, and as an eye drop.

Rifabutin (Rfb) is an antibiotic used to treat tuberculosis and prevent and treat Mycobacterium avium complex. It is typically only used in those who cannot tolerate rifampin such as people with HIV/AIDS on antiretrovirals. For active tuberculosis it is used with other antimycobacterial medications. For latent tuberculosis it may be used by itself when the exposure was with drug-resistant TB.

Rifapentine, sold under the brand name Priftin, is an antibiotic used in the treatment of tuberculosis. In active tuberculosis it is used together with other antituberculosis medications. In latent tuberculosis it is typically used with isoniazid. It is taken by mouth.
TB Alliance is a not-for-profit product development partnership (PDP) dedicated to the discovery and development of new, faster-acting and affordable tuberculosis (TB) medicines. Since its inception in 2000, TB Alliance has worked to grow the field of available treatments for TB and now manages the largest pipeline of new TB drugs in history. It was founded in Cape Town, South Africa, and has since expanded. It is headquartered in New York City and has a regional office in Pretoria.
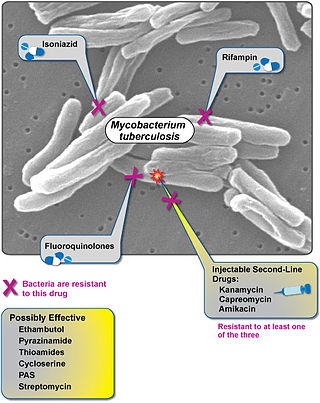
Extensively drug-resistant tuberculosis (XDR-TB) is a form of tuberculosis caused by bacteria that are resistant to some of the most effective anti-TB drugs. XDR-TB strains have arisen after the mismanagement of individuals with multidrug-resistant TB (MDR-TB).
Multidrug-resistant tuberculosis (MDR-TB) is a form of tuberculosis (TB) infection caused by bacteria that are resistant to treatment with at least two of the most powerful first-line anti-TB medications (drugs): isoniazid and rifampin. Some forms of TB are also resistant to second-line medications, and are called extensively drug-resistant TB (XDR-TB).
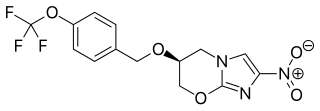
Pretomanid is an antibiotic medication used for the treatment of multi-drug-resistant tuberculosis affecting the lungs. It is generally used together with bedaquiline and linezolid. It is taken by mouth.
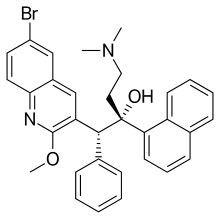
Koen Andries is a Belgian Janssen Pharmaceutica scientist and professor at the University of Antwerp. In 2005 he and his team published a discovery about a new di-Aryl-Quinoline-based drug (R207910), now called bedaquiline, which promises a shorter and simpler treatment for drug resistant Tuberculosis (TB).
Delamanid, sold under the brand name Deltyba, is a medication used to treat tuberculosis. Specifically it is used, along with other antituberculosis medications, for active multidrug-resistant tuberculosis. It is taken by mouth.
Diaryl quinolines (DARQs) are a chemical class of drugs that treat tuberculosis. They target subunit c of mycobacterial ATP synthase, inhibiting the enzyme so mycobacterium tuberculosis cannot synthesise ATP. This effectively kills the bacteria.

Ceftazidime/avibactam, sold under the brand name Avycaz among others, is a fixed-dose combination medication composed of ceftazidime, a cephalosporin antibiotic, and avibactam, a β-lactamase inhibitor. It is used to treat complicated intra-abdominal infections, urinary tract infections, and pneumonia. It is only recommended when other options are not appropriate. It is given by injection into a vein.

Lefamulin, sold under the brand name Xenleta, is an antibiotic medication used it to treat adults with community-acquired bacterial pneumonia. It is taken by mouth or by injection into a vein.

Anil Koul is a scientist and former Director of the CSIR-Institute of Microbial Technology (IMTECH), a premier biomedical and biotechnology research institution under Council of Scientific and Industrial Research (CSIR) under Ministry of Science and Technology, Govt. of India.

Lenacapavir, sold under the brand name Sunlenca, is an antiretroviral medication used to treat HIV/AIDS. It is taken by mouth or by subcutaneous injection.