
Cytarabine, also known as cytosine arabinoside (ara-C), is a chemotherapy medication used to treat acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), chronic myelogenous leukemia (CML), and non-Hodgkin's lymphoma. It is given by injection into a vein, under the skin, or into the cerebrospinal fluid. There is a liposomal formulation for which there is tentative evidence of better outcomes in lymphoma involving the meninges.
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone and methylene groups: [−C(=O)−CH2−]n. First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity.

Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.
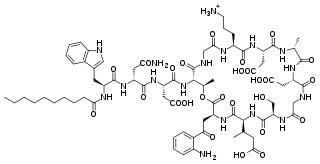
Daptomycin, sold under the brand name Cubicin among others, is a lipopeptide antibiotic used in the treatment of systemic and life-threatening infections caused by Gram-positive organisms.

Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis activity. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified, and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin. The tuberactinomycins target bacterial ribosomes, binding RNA and disrupting bacterial protein synthesis and certain forms of RNA splicing. Viomycin is produced by the actinomycete Streptomyces puniceus.

Activation-induced cytidine deaminase, also known as AICDA, AID and single-stranded DNA cytosine deaminase, is a 24 kDa enzyme which in humans is encoded by the AICDA gene. It creates mutations in DNA by deamination of cytosine base, which turns it into uracil. In other words, it changes a C:G base pair into a U:G mismatch. The cell's DNA replication machinery recognizes the U as a T, and hence C:G is converted to a T:A base pair. During germinal center development of B lymphocytes, AID also generates other types of mutations, such as C:G to A:T. The mechanism by which these other mutations are created is not well understood. It is a member of the APOBEC family.

Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics. Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite that is one of the most abundantly produced metabolites in human invasive Aspergillosis (IA).

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.
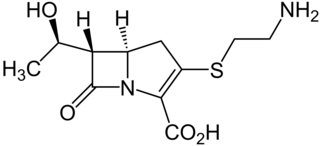
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.
In enzymology, a blasticidin-S deaminase (EC 3.5.4.23) is an enzyme that catalyzes the chemical reaction

Apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1 also known as C->U-editing enzyme APOBEC-1 is a protein that in humans is encoded by the APOBEC1 gene.

Cytidine deaminase is an enzyme that in humans is encoded by the CDA gene.

Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3A, also known as APOBEC3A, or A3A is a gene of the APOBEC3 family found in humans, non-human primates, and some other mammals. It is a single-domain DNA cytidine deaminase with antiviral effects. While other members of the family such as APOBEC3G are believed to act by editing ssDNA by removing an amino group from cytosine in DNA, introducing a cytosine to uracil change which can ultimately lead to a cytosine to thymine mutation, one study suggests that APOBEC3A can inhibit parvoviruses by another mechanism. The cellular function of APOBEC3A is likely to be the destruction of foreign DNA through extensive deamination of cytosine.Stenglein MD, Burns MB, Li M, Lengyel J, Harris RS. "APOBEC3 proteins mediate the clearance of foreign DNA from human cells". Nature Structural & Molecular Biology. 17 (2): 222–9. doi:10.1038/nsmb.1744. PMC 2921484. PMID 20062055.

Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as Streptomyces azureus and Streptomyces laurentii. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class.
Streptogramin A is a group of antibiotics within the larger family of antibiotics known as streptogramins. They are synthesized by the bacteria Streptomyces virginiae. The streptogramin family of antibiotics consists of two distinct groups: group A antibiotics contain a 23-membered unsaturated ring with lactone and peptide bonds while group B antibiotics are depsipeptides. While structurally different, these two groups of antibiotics act synergistically, providing greater antibiotic activity than the combined activity of the separate components. These antibiotics have until recently been commercially manufactured as feed additives in agriculture, although today there is increased interest in their ability to combat antibiotic-resistant bacteria, particularly vancomycin-resistant bacteria.
Streptogramin B is a subgroup of the streptogramin antibiotics family. These natural products are cyclic hexa- or hepta depsipeptides produced by various members of the genus of bacteria Streptomyces. Many of the members of the streptogramins reported in the literature have the same structure and different names; for example, pristinamycin IA = vernamycin Bα = mikamycin B = osteogrycin B.
Radical SAMenzymes is a superfamily of enzymes that use a [4Fe-4S]+ cluster to reductively cleave S-adenosyl-L-methionine (SAM) to generate a radical, usually a 5′-deoxyadenosyl radical (5'-dAdo), as a critical intermediate. These enzymes utilize this radical intermediate to perform diverse transformations, often to functionalize unactivated C-H bonds. Radical SAM enzymes are involved in cofactor biosynthesis, enzyme activation, peptide modification, post-transcriptional and post-translational modifications, metalloprotein cluster formation, tRNA modification, lipid metabolism, biosynthesis of antibiotics and natural products etc. The vast majority of known radical SAM enzymes belong to the radical SAM superfamily, and have a cysteine-rich motif that matches or resembles CxxxCxxC. Radical SAM enzymes comprise the largest superfamily of metal-containing enzymes.

Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from Streptomyces bottropensis. It has been shown to inhibit methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) among other Gram-positive bacteria and mycoplasma. Bottromycin is structurally distinct from both vancomycin, a glycopeptide antibiotic, and methicillin, a beta-lactam antibiotic.
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions.

Nosiheptide is a thiopeptide antibiotic produced by the bacterium Streptomyces actuosus.














