The putamen is a round structure located at the base of the forebrain (telencephalon). The putamen and caudate nucleus together form the dorsal striatum. It is also one of the structures that compose the basal nuclei. Through various pathways, the putamen is connected to the substantia nigra, the globus pallidus, the claustrum, and the thalamus, in addition to many regions of the cerebral cortex. A primary function of the putamen is to regulate movements at various stages and influence various types of learning. It employs GABA, acetylcholine, and enkephalin to perform its functions. The putamen also plays a role in degenerative neurological disorders, such as Parkinson's disease.

The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

Dementia with Lewy bodies (DLB) is a type of dementia characterized by changes in sleep, behavior, cognition, movement, and regulation of automatic bodily functions. Memory loss is not always an early symptom. The disease worsens over time and is usually diagnosed when cognitive impairment interferes with normal daily functioning. Together with Parkinson's disease dementia, DLB is one of the two Lewy body dementias. It is a common form of dementia, but the prevalence is not known accurately and many diagnoses are missed. The disease was first described by Kenji Kosaka in 1976.

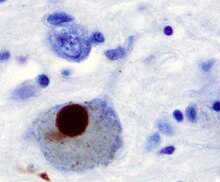
Lewy bodies are the inclusion bodies – abnormal aggregations of protein – that develop inside nerve cells affected by Parkinson's disease (PD), the Lewy body dementias, and some other disorders. They are also seen in cases of multiple system atrophy, particularly the parkinsonian variant (MSA-P).

MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an organic compound. It is classified as a tetrahydropyridine. It is of interest as a precursor to the neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animals.

The nigrostriatal pathway is a bilateral dopaminergic pathway in the brain that connects the substantia nigra pars compacta (SNc) in the midbrain with the dorsal striatum in the forebrain. It is one of the four major dopamine pathways in the brain, and is critical in the production of movement as part of a system called the basal ganglia motor loop. Dopaminergic neurons of this pathway release dopamine from axon terminals that synapse onto GABAergic medium spiny neurons (MSNs), also known as spiny projection neurons (SPNs), located in the striatum.

Neuromelanin (NM) is a dark pigment found in the brain which is structurally related to melanin. It is a polymer of 5,6-dihydroxyindole monomers. Neuromelanin is found in large quantities in catecholaminergic cells of the substantia nigra pars compacta and locus coeruleus, giving a dark color to the structures.
Hypokinesia is one of the classifications of movement disorders, and refers to decreased bodily movement. Hypokinesia is characterized by a partial or complete loss of muscle movement due to a disruption in the basal ganglia. Hypokinesia is a symptom of Parkinson's disease shown as muscle rigidity and an inability to produce movement. It is also associated with mental health disorders and prolonged inactivity due to illness, amongst other diseases.
The pedunculopontine nucleus (PPN) or pedunculopontine tegmental nucleus is a collection of neurons located in the upper pons in the brainstem. It lies caudal to the substantia nigra and adjacent to the superior cerebellar peduncle. It has two divisions of subnuclei; the pars compacta containing mainly cholinergic neurons, and the pars dissipata containing mainly glutamatergic neurons and some non-cholinergic neurons. The pedunculopontine nucleus is one of the main components of the reticular activating system. It was first described in 1909 by Louis Jacobsohn-Lask, a German neuroanatomist.
The pars compacta (SNpc) is one of two subdivisions of the substantia nigra of the midbrain ; it is situated medial to the pars reticulata. It is formed by dopaminergic neurons. It projects to the striatum and portions of the cerebral cortex. It is functionally involved in fine motor control.

Beta-synuclein is a protein that in humans is encoded by the SNCB gene.

Vacuolar protein sorting ortholog 35 (VPS35) is a protein involved in autophagy and is implicated in neurodegenerative diseases, such as Parkinson's disease (PD) and Alzheimer's disease (AD). VPS35 is part of a complex called the retromer, which is responsible for transporting select cargo proteins between vesicular structures and the Golgi apparatus. Mutations in the VPS35 gene (VPS35) cause aberrant autophagy, where cargo proteins fail to be transported and dysfunctional or unnecessary proteins fail to be degraded. There are numerous pathways affected by altered VPS35 levels and activity, which have clinical significance in neurodegeneration. There is therapeutic relevance for VPS35, as interventions aimed at correcting VPS35 function are in speculation.

Parkinson's disease (PD), or simply Parkinson's, is a chronic degenerative disorder of the central nervous system that affects both the motor system and non-motor systems. The symptoms usually emerge slowly, and as the disease progresses, non-motor symptoms become more common. Early symptoms are tremor, rigidity, slowness of movement, and difficulty with walking. Problems may also arise with cognition, behaviour, sleep, and sensory systems. Parkinson's disease dementia is common in advanced stages.

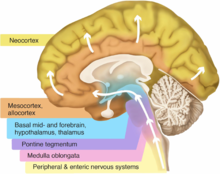
Basal ganglia disease is a group of physical problems that occur when the group of nuclei in the brain known as the basal ganglia fail to properly suppress unwanted movements or to properly prime upper motor neuron circuits to initiate motor function. Research indicates that increased output of the basal ganglia inhibits thalamocortical projection neurons. Proper activation or deactivation of these neurons is an integral component for proper movement. If something causes too much basal ganglia output, then the ventral anterior (VA) and ventral lateral (VL) thalamocortical projection neurons become too inhibited, and one cannot initiate voluntary movement. These disorders are known as hypokinetic disorders. However, a disorder leading to abnormally low output of the basal ganglia leads to reduced inhibition, and thus excitation, of the thalamocortical projection neurons which synapse onto the cortex. This situation leads to an inability to suppress unwanted movements. These disorders are known as hyperkinetic disorders.

The history of Parkinson's disease expands from 1817, when British apothecary James Parkinson published An Essay on the Shaking Palsy, to modern times. Before Parkinson's descriptions, others had already described features of the disease that would bear his name, while the 20th century greatly improved knowledge of the disease and its treatments. PD was then known as paralysis agitans. The term "Parkinson's disease" was coined in 1865 by William Sanders and later popularized by French neurologist Jean-Martin Charcot. Paralysis
Gene therapy in Parkinson's disease consists of the creation of new cells that produce a specific neurotransmitter (dopamine), protect the neural system, or the modification of genes that are related to the disease. Then these cells are transplanted to a patient with the disease. There are different kinds of treatments that focus on reducing the symptoms of the disease but currently there is no cure.
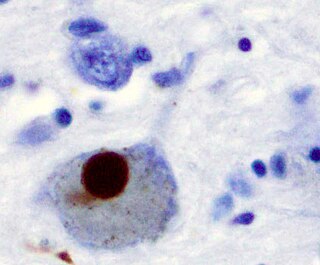
Synucleinopathies are neurodegenerative diseases characterised by the abnormal accumulation of aggregates of alpha-synuclein protein in neurons, nerve fibres or glial cells. There are three main types of synucleinopathy: Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). Other rare disorders, such as various neuroaxonal dystrophies, also have α-synuclein pathologies. Additionally, autopsy studies have shown that around 6% of sporadic Alzheimer's Disease exhibit α-synuclein positive Lewy pathology, and are sub-classed as Alzheimer's Disease with Amygdalar Restricted Lewy Bodies (AD/ALB).

The pathophysiology of Parkinson's disease is death of dopaminergic neurons as a result of changes in biological activity in the brain with respect to Parkinson's disease (PD). There are several proposed mechanisms for neuronal death in PD; however, not all of them are well understood. Five proposed major mechanisms for neuronal death in Parkinson's Disease include protein aggregation in Lewy bodies, disruption of autophagy, changes in cell metabolism or mitochondrial function, neuroinflammation, and blood–brain barrier (BBB) breakdown resulting in vascular leakiness.

Animal models of Parkinson's disease are essential in the research field and widely used to study Parkinson's disease. Parkinson's disease is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of the dopamine neurons in the brain, results in motor dysfunction, ultimately causing the four cardinal symptoms of PD: tremor, rigidity, postural instability, and bradykinesia. It is the second most prevalent neurodegenerative disease, following Alzheimer's disease. It is estimated that nearly one million people could be living with PD in the United States.
Rapid eye movement sleep behaviour disorder and Parkinson's disease is rapid eye movement sleep behavior disorder (RBD) that is associated with Parkinson's disease. RBC is linked genetically and neuropathologically to α- synuclein, a presynaptic neuronal protein that exerts deleterious effects on neighbouring proteins, leading to neuronal death. This pathology is linked to numerous other neurodegenerative disorders, such as Lewy bodies dementia, and collectively these disorders are known as synucleinopathies. Numerous reports over the past few years have stated the frequent association of synucleinopathies with REM sleep behaviour disorder (RBD). In particular, the frequent association of RBD with Parkinson's. In the general population the incidence of RBD is around 0.5%, compared to the prevalence of RBD in PD patients, which has been reported to be between 38% and 60%. The diagnosis and symptom onset of RBD typically precedes the onset of motor or cognitive symptoms of PD by a number of years, typically ranging anywhere from 2 to 15 years prior. Hence, this link could provide an important window of opportunity in the implementation of therapies and treatments, that could prevent or slow the onset of PD.