
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.

The endomembrane system is composed of the different membranes (endomembranes) that are suspended in the cytoplasm within a eukaryotic cell. These membranes divide the cell into functional and structural compartments, or organelles. In eukaryotes the organelles of the endomembrane system include: the nuclear membrane, the endoplasmic reticulum, the Golgi apparatus, lysosomes, vesicles, endosomes, and plasma (cell) membrane among others. The system is defined more accurately as the set of membranes that forms a single functional and developmental unit, either being connected directly, or exchanging material through vesicle transport. Importantly, the endomembrane system does not include the membranes of plastids or mitochondria, but might have evolved partially from the actions of the latter.

The Golgi apparatus, also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus.

In cell biology, a vesicle is a structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer. Vesicles form naturally during the processes of secretion (exocytosis), uptake (endocytosis), and the transport of materials within the plasma membrane. Alternatively, they may be prepared artificially, in which case they are called liposomes. If there is only one phospholipid bilayer, the vesicles are called unilamellar liposomes; otherwise they are called multilamellar liposomes. The membrane enclosing the vesicle is also a lamellar phase, similar to that of the plasma membrane, and intracellular vesicles can fuse with the plasma membrane to release their contents outside the cell. Vesicles can also fuse with other organelles within the cell. A vesicle released from the cell is known as an extracellular vesicle.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.

COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is retrograde transport, in contrast to the anterograde transport associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the cis-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ.

The vesicular-tubular cluster (VTC), also referred to as the endoplasmic-reticulum–Golgi intermediate compartment (ERGIC), is an organelle in eukaryotic cells. This compartment mediates trafficking between the endoplasmic reticulum (ER) and Golgi complex, facilitating the sorting of cargo. The cluster was first identified in 1988 using an antibody to the protein that has since been named ERGIC-53.
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known:

General vesicular transport factor p115 is a protein that in humans is encoded by the USO1 gene.

Syntaxin-5 is a protein that in humans is encoded by the STX5 gene.

KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1, also known as KDELR1, is a protein which in humans is encoded by the KDELR1 gene.

VAMP-Associated Protein A is a protein that in humans is encoded by the VAPA gene. Together with VAPB and VAPC it forms the VAP protein family. They are integral endoplasmic reticulum membrane proteins of the type II and are ubiquitous among eukaryotes.
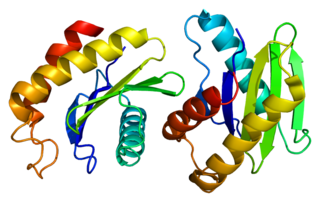
Vesicle-trafficking protein SEC22b is a protein that in humans is encoded by the SEC22B gene.

BET1 homolog is a protein that in humans is encoded by the BET1 gene.

Oxysterol-binding protein 1 is a protein that in humans is encoded by the OSBP gene.

Golgi SNAP receptor complex member 2 is a protein that in humans is encoded by the GOSR2 gene.
KDEL is a target peptide sequence in mammals and plants located on the C-terminal end of the amino acid structure of a protein. The KDEL sequence prevents a protein from being secreted from the endoplasmic reticulum (ER) and facilitates its return if it is accidentally exported.

Jennifer Lippincott-Schwartz is a Senior Group Leader at Howard Hughes Medical Institute's Janelia Research Campus and a founding member of the Neuronal Cell Biology Program at Janelia. Previously, she was the Chief of the Section on Organelle Biology in the Cell Biology and Metabolism Program, in the Division of Intramural Research in the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health from 1993 to 2016. Lippincott-Schwartz received her PhD from Johns Hopkins University, and performed post-doctoral training with Richard Klausner at the NICHD, NIH in Bethesda, Maryland.
Membrane vesicle trafficking in eukaryotic animal cells involves movement of biochemical signal molecules from synthesis-and-packaging locations in the Golgi body to specific release locations on the inside of the plasma membrane of the secretory cell. It takes place in the form of Golgi membrane-bound micro-sized vesicles, termed membrane vesicles (MVs).
Modeccin is a toxic lectin, a group of glycoproteins capable of binding specifically to sugar moieties. Different toxic lectins are present in seeds of different origin. Modeccin is found in the roots of the African plant Adenia digitata. These roots are often mistaken for edible roots, which has led to some cases of intoxication. Sometimes the fruit is eaten, or a root extract is drunk as a manner of suicide.















