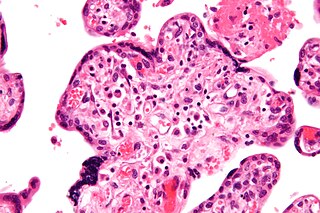
Intrauterine growth restriction (IUGR), or fetal growth restriction, is the poor growth of a fetus while in the womb during pregnancy. IUGR is defined by clinical features of malnutrition and evidence of reduced growth regardless of an infant's birth weight percentile. The causes of IUGR are broad and may involve maternal, fetal, or placental complications.

Preterm birth, also known as premature birth, is the birth of a baby at fewer than 37 weeks gestational age, as opposed to full-term delivery at approximately 40 weeks. Extreme preterm is less than 28 weeks, very early preterm birth is between 28 and 32 weeks, early preterm birth occurs between 32 and 34 weeks, late preterm birth is between 34 and 36 weeks' gestation. These babies are also known as premature babies or colloquially preemies or premmies. Symptoms of preterm labor include uterine contractions which occur more often than every ten minutes and/or the leaking of fluid from the vagina before 37 weeks. Premature infants are at greater risk for cerebral palsy, delays in development, hearing problems and problems with their vision. The earlier a baby is born, the greater these risks will be.

Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen at increased partial pressures. Severe cases can result in cell damage and death, with effects most often seen in the central nervous system, lungs, and eyes. Historically, the central nervous system condition was called the Paul Bert effect, and the pulmonary condition the Lorrain Smith effect, after the researchers who pioneered the discoveries and descriptions in the late 19th century. Oxygen toxicity is a concern for underwater divers, those on high concentrations of supplemental oxygen, and those undergoing hyperbaric oxygen therapy.
Retinopathy of prematurity (ROP), also called retrolental fibroplasia (RLF) and Terry syndrome, is a disease of the eye affecting prematurely born babies generally having received neonatal intensive care, in which oxygen therapy is used because of the premature development of their lungs. It is thought to be caused by disorganized growth of retinal blood vessels and may result in scarring and retinal detachment. ROP can be mild and may resolve spontaneously, but it may lead to blindness in serious cases. Thus, all preterm babies are at risk for ROP, and very low birth-weight is an additional risk factor. Both oxygen toxicity and relative hypoxia can contribute to the development of ROP.

Infant respiratory distress syndrome (IRDS), also called respiratory distress syndrome of newborn, or increasingly surfactant deficiency disorder (SDD), and previously called hyaline membrane disease (HMD), is a syndrome in premature infants caused by developmental insufficiency of pulmonary surfactant production and structural immaturity in the lungs. It can also be a consequence of neonatal infection and can result from a genetic problem with the production of surfactant-associated proteins.
Transient tachypnea of the newborn is a respiratory problem that can be seen in the newborn shortly after delivery. It is caused by retained fetal lung fluid due to impaired clearance mechanisms. It is the most common cause of respiratory distress in term neonates. It consists of a period of tachypnea (rapid breathing. Usually, this condition resolves over 24–72 hours. Treatment is supportive and may include supplemental oxygen and antibiotics. The chest x-ray shows hyperinflation of the lungs including prominent pulmonary vascular markings, flattening of the diaphragm, and fluid in the horizontal fissure of the right lung.

A neonatal intensive care unit (NICU), also known as an intensive care nursery (ICN), is an intensive care unit (ICU) specializing in the care of ill or premature newborn infants. The NICU is divided into several areas, including a critical care area for babies who require close monitoring and intervention, an intermediate care area for infants who are stable but still require specialized care, and a step down unit where babies who are ready to leave the hospital can receive additional care before being discharged.
Antenatal steroids, also known as antenatal corticosteroids, are medications administered to pregnant women expecting a preterm birth. When administered, these steroids accelerate the maturation of the fetus' lungs, which reduces the likelihood of infant respiratory distress syndrome and infant mortality. The effectiveness of this corticosteroid treatment on humans was first demonstrated in 1972 by Sir Graham Liggins and Ross Howie, during a randomized control trial using betamethasone.
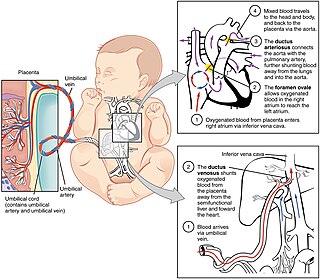
In humans, the circulatory system is different before and after birth. The fetal circulation is composed of the placenta, umbilical blood vessels encapsulated by the umbilical cord, heart and systemic blood vessels. A major difference between the fetal circulation and postnatal circulation is that the lungs are not used during the fetal stage resulting in the presence of shunts to move oxygenated blood and nutrients from the placenta to the fetal tissue. At birth, the start of breathing and the severance of the umbilical cord prompt various changes that quickly transform fetal circulation into postnatal circulation.

Respiratory diseases, or lung diseases, are pathological conditions affecting the organs and tissues that make gas exchange difficult in air-breathing animals. They include conditions of the respiratory tract including the trachea, bronchi, bronchioles, alveoli, pleurae, pleural cavity, the nerves and muscles of respiration. Respiratory diseases range from mild and self-limiting, such as the common cold, influenza, and pharyngitis to life-threatening diseases such as bacterial pneumonia, pulmonary embolism, tuberculosis, acute asthma, lung cancer, and severe acute respiratory syndromes, such as COVID-19. Respiratory diseases can be classified in many different ways, including by the organ or tissue involved, by the type and pattern of associated signs and symptoms, or by the cause of the disease.

An analeptic, in medicine, is a central nervous system stimulant. The term "analeptic" typically refers to respiratory stimulants. Analeptics are central nervous system (CNS) stimulants that include a wide variety of medications used to treat depression, attention deficit hyperactivity disorder (ADHD), and respiratory depression. Analeptics can also be used as convulsants, with low doses causing patients to experience heightened awareness, restlessness, and rapid breathing. The primary medical use of these drugs is as an anesthetic recovery tool or to treat emergency respiratory depression. Other drugs of this category are prethcamide, pentylenetetrazole, and nikethamide. Nikethamide is now withdrawn due to risk of convulsions. Analeptics have recently been used to better understand the treatment of a barbiturate overdose. Through the use of agents, researchers were able to treat obtundation and respiratory depression.
Palivizumab, sold under the brand name Synagis, is a monoclonal antibody produced by recombinant DNA technology used to prevent severe disease caused by respiratory syncytial virus (RSV) infections. It is recommended for infants at high-risk for RSV due to conditions such as prematurity or other medical problems including heart or lung diseases.
Wilson–Mikity syndrome, a form of chronic lung disease (CLD) that exists only in premature infants, leads to progressive or immediate development of respiratory distress. This rare condition affects low birth babies and is characterized by rapid development of lung emphysema after birth, requiring prolonged ventilation and oxygen supplementation. It is closely related to bronchopulmonary dysplasia (BPD), differing mainly in the lack of prior ventilatory support. All the initial patients described with Wilson–Mikity syndrome were very low birth weight infants that had no history of mechanical ventilation, yet developed a syndrome that clinically resembled BPD. Upon the death of some of these infants, autopsies showed histologic changes similar to those seen in BPD.
Persistent fetal circulation is a condition caused by a failure in the systemic circulation and pulmonary circulation to convert from the antenatal circulation pattern to the "normal" pattern. Infants experience a high mean arterial pulmonary artery pressure and a high afterload at the right ventricle. This means that the heart is working against higher pressures, which makes it more difficult for the heart to pump blood.
Alveolar capillary dysplasia (ACD) is a rare, congenital diffuse lung disease characterized by abnormal blood vessels in the lungs that cause highly elevated pulmonary blood pressure and an inability to effectively oxygenate and remove carbon dioxide from the blood. ACD typically presents in newborn babies within hours of birth as rapid and labored breathing, blue-colored lips or skin, quickly leading to respiratory failure and death. Atypical forms of ACD have been reported with initially milder symptoms and survival of many months before the onset of respiratory failure or lung transplantation.

Pulmonary interstitial emphysema (PIE) is a collection of air outside of the normal air space of the pulmonary alveoli, found instead inside the connective tissue of the peribronchovascular sheaths, interlobular septa, and visceral pleura. This collection of air develops as a result of alveolar and terminal bronchiolar rupture. Pulmonary interstitial emphysema is more frequent in premature infants who require mechanical ventilation for severe lung disease. Infants with pulmonary interstitial emphysema are typically recommended for admission to a neonatal intensive care unit.
Bubble CPAP is a non-invasive ventilation strategy for newborns with infant respiratory distress syndrome (IRDS). It is one of the methods by which continuous positive airway pressure (CPAP) is delivered to a spontaneously breathing newborn to maintain lung volumes during expiration. With this method, blended and humidified oxygen is delivered via short binasal prongs or a nasal mask and pressure in the circuit is maintained by immersing the distal end of the expiratory tubing in water. The depth to which the tubing is immersed underwater determines the pressure generated in the airways of the infant. As the gas flows through the system, it "bubbles" out and prevents buildup of excess pressures.
Surfactant therapy is the medical administration of exogenous surfactant. Surfactants used in this manner are typically instilled directly into the trachea. When a baby comes out of the womb and the lungs are not developed yet, they require administration of surfactant in order to process oxygen and survive. This condition that the baby has is called newborn respiratory distress syndrome, and it is treatable. Surfactant coat the smallest parts of the lungs called the alveoli and helps for oxygen to go in and for carbon dioxide to go out. How surfactant does this is by not allowing the alveoli to collapse and to retain their inflated shape when the baby exhales.
Christian P. Speer is a German pediatrician and Professor of Pediatrics specialized in neonatology at the Julius Maximilian University of Würzburg. Speer is known for his scientific and educational contributions in neonatal medicine.

Henry Lewis Halliday was a British-Irish paediatrician and neonatologist. In 2021, Halliday was awarded the James Spence Medal for research into neonatology, for coordinating two of the largest neonatal multicentre trials for prevention and treatment of a number of neonatal respiratory illnesses and for a breakthrough in the development of a new lung surfactant that brought relief to very small babies suffering from infant respiratory distress syndrome (RDS).










