Related Research Articles
Developmental biology is the study of the process by which animals and plants grow and develop. Developmental biology also encompasses the biology of regeneration, asexual reproduction, metamorphosis, and the growth and differentiation of stem cells in the adult organism.
Morphogenesis is the biological process that causes a cell, tissue or organism to develop its shape. It is one of three fundamental aspects of developmental biology along with the control of tissue growth and patterning of cellular differentiation.
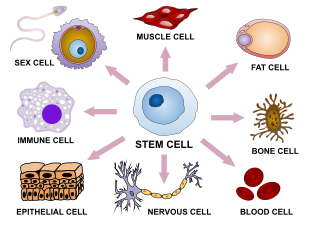
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. However, metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.

In the developing chordate, the neural tube is the embryonic precursor to the central nervous system, which is made up of the brain and spinal cord. The neural groove gradually deepens as the neural fold become elevated, and ultimately the folds meet and coalesce in the middle line and convert the groove into the closed neural tube. In humans, neural tube closure usually occurs by the fourth week of pregnancy.
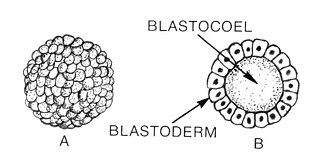
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.
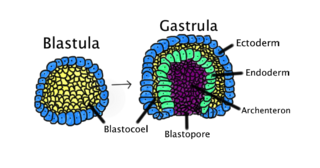
Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. In the late blastocyst, the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother.

Drosophila embryogenesis, the process by which Drosophila embryos form, is a favorite model system for genetics and developmental biology. The study of its embryogenesis unlocked the century-long puzzle of how development was controlled, creating the field of evolutionary developmental biology. The small size, short generation time, and large brood size make it ideal for genetic studies. Transparent embryos facilitate developmental studies. Drosophila melanogaster was introduced into the field of genetic experiments by Thomas Hunt Morgan in 1909.
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.

A morphogen is a substance whose non-uniform distribution governs the pattern of tissue development in the process of morphogenesis or pattern formation, one of the core processes of developmental biology, establishing positions of the various specialized cell types within a tissue. More specifically, a morphogen is a signaling molecule that acts directly on cells to produce specific cellular responses depending on its local concentration.

Neural crest cells are a temporary group of cells that arise from the embryonic ectoderm germ layer, and in turn give rise to a diverse cell lineage—including melanocytes, craniofacial cartilage and bone, smooth muscle, peripheral and enteric neurons and glia.
Gametogonium are stem cells for gametes located within the gonads. They originate from primordial germ cells, which have migrated to the gonads. Male gametogonia which are located within the testes during development and adulthood are called spermatogonium. Female gametogonia, known as oogonium, are found within the ovaries of the developing foetus and were thought to be depleted at or after birth. Spermatogonia and oogonia are classified as sexually differentiated germ cells.
Decapentaplegic (Dpp) is a key morphogen involved in the development of the fruit fly Drosophila melanogaster and is the first validated secreted morphogen. It is known to be necessary for the correct patterning and development of the early Drosophila embryo and the fifteen imaginal discs, which are tissues that will become limbs and other organs and structures in the adult fly. It has also been suggested that Dpp plays a role in regulating the growth and size of tissues. Flies with mutations in decapentaplegic fail to form these structures correctly, hence the name. Dpp is the Drosophila homolog of the vertebrate bone morphogenetic proteins (BMPs), which are members of the TGF-β superfamily, a class of proteins that are often associated with their own specific signaling pathway. Studies of Dpp in Drosophila have led to greater understanding of the function and importance of their homologs in vertebrates like humans.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

The French flag model is a conceptual definition of a morphogen, described by Lewis Wolpert in the 1960s. A morphogen is defined as a signaling molecule that acts directly on cells to produce specific cellular responses dependent on morphogen concentration. During early development, morphogen gradients generate different cell types in distinct spatial order. French flag patterning is often found in combination with others: vertebrate limb development is one of the many phenotypes exhibiting French flag patterning overlapped with a complementary pattern.

Osteochondroprogenitor cells are progenitor cells that arise from mesenchymal stem cells (MSC) in the bone marrow. They have the ability to differentiate into osteoblasts or chondrocytes depending on the signalling molecules they are exposed to, giving rise to either bone or cartilage respectively. Osteochondroprogenitor cells are important for bone formation and maintenance.
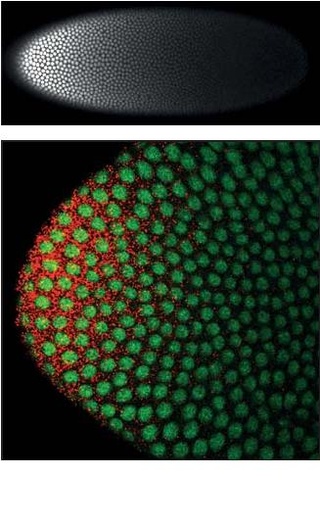
Homeotic protein bicoid is encoded by the bcd maternal effect gene in Drosophilia. Homeotic protein bicoid concentration gradient patterns the anterior-posterior (A-P) axis during Drosophila embryogenesis. Bicoid was the first protein demonstrated to act as a morphogen. Although bicoid is important for the development of Drosophila and other higher dipterans, it is absent from most other insects, where its role is accomplished by other genes.
Magdalena Żernicka-Goetz is a Polish-British developmental biologist. She is Professor of Mammalian Development and Stem Cell Biology in the Department of Physiology, Development and Neuroscience and Fellow of Sidney Sussex College, Cambridge. She also serves as Bren Professor of Biology and Biological Engineering at California Institute of Technology (Caltech).
The Spemann-Mangold organizer is a group of cells that are responsible for the induction of the neural tissues during development in amphibian embryos. First described in 1924 by Hans Spemann and Hilde Mangold, the introduction of the organizer provided evidence that the fate of cells can be influenced by factors from other cell populations. This discovery significantly impacted the world of developmental biology and fundamentally changed the understanding of early development.

The dorsal lip of the blastopore is a structure that forms during early embryonic development and is important for its role in organizing the germ layers. The dorsal lip is formed during early gastrulation as folding of tissue along the involuting marginal zone of the blastocoel forms an opening known as the blastopore. It is particularly important for its role in neural induction through the default model, where signaling from the dorsal lip protects a region of the epiblast from becoming epidermis, thus allowing it to develop to its default neural tissue.
References
- ↑ Wallingford, John B; Fraser, Scott E; Harland, Richard M (2002-06-01). "Convergent Extension: The Molecular Control of Polarized Cell Movement during Embryonic Development". Developmental Cell. 2 (6): 695–706. doi: 10.1016/S1534-5807(02)00197-1 . ISSN 1534-5807. PMID 12062082.
- ↑ Miura, Masayuki; Yamaguchi, Yoshifumi (2015-02-23). "Programmed Cell Death in Neurodevelopment". Developmental Cell. 32 (4): 478–490. doi: 10.1016/j.devcel.2015.01.019 . ISSN 1534-5807. PMID 25710534.
- ↑ Ranganath, R. M.; Nagashree, N. R. (2001). "Role of programmed cell death in development". International Review of Cytology. 202: 159–242. doi:10.1016/s0074-7696(01)02005-8. ISBN 9780123646064. ISSN 0074-7696. PMID 11061565.
- ↑ Saenko, SV; French, V; Brakefield, PM; Beldade, P (27 April 2008). "Conserved developmental processes and the formation of evolutionary novelties: examples from butterfly wings". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 363 (1496): 1549–55. doi:10.1098/rstb.2007.2245. PMC 2615821 . PMID 18192179.
- ↑ Streuli, Charles H. (2009-01-15). "Integrins and cell-fate determination". Journal of Cell Science. 122 (2): 171–177. doi: 10.1242/jcs.018945 . ISSN 0021-9533. PMC 2714415 . PMID 19118209.
- ↑ Featherstone, D. E.; Broadie, K. S. (2005-01-01), Gilbert, Lawrence I. (ed.), "2.3 - Functional Development of the Neuromusculature", Comprehensive Molecular Insect Science, Amsterdam: Elsevier, pp. 85–134, ISBN 978-0-444-51924-5 , retrieved 2021-03-22
- ↑ Dev Dyn 2010, 239:1315-1329. Maduro, M. F. (2010). "Cell fate specification in the C. Elegans embryo". Developmental Dynamics. 239 (5): 1315–1329. doi: 10.1002/dvdy.22233 . PMID 20108317. S2CID 14633229.
- ↑ Zernicka-Goetz M: First cell fate decisions and spatial patterning in the early mouse embryo. Semin Cell Dev Biol 2004, 15:563-572.Zernicka-Goetz, M. (2004). "First cell fate decisions and spatial patterning in the early mouse embryo". Seminars in Cell & Developmental Biology. 15 (5): 563–572. doi:10.1016/j.semcdb.2004.04.004. PMID 15271302.
- ↑ Artavanis-Tsakonas S, Rand MD, Lake RJ: Notch signaling: cell fate control and signal integration in development. Science 1999, 284:770-776.Artavanis-Tsakonas, S.; Rand, M. D.; Lake, R. J. (1999). "Notch Signaling: Cell Fate Control and Signal Integration in Development". Science. 284 (5415): 770–6. Bibcode:1999Sci...284..770A. doi:10.1126/science.284.5415.770. PMID 10221902.
- ↑ Schuurmans C, Guillemot F: Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol 2002, 12:26-34.Schuurmans, C.; Guillemot, F. (2002). "Molecular mechanisms underlying cell fate specification in the developing telencephalon". Current Opinion in Neurobiology. 12 (1): 26–34. doi:10.1016/S0959-4388(02)00286-6. PMID 11861161. S2CID 27988180.
- ↑ Rohrschneider MR, Nance J: Polarity and cell fate specification in the control of Caenorhabditis elegans gastrulation. Dev Dyn 2009, 238:789-796. Rohrschneider, M.; Nance, J. (2009). "Polarity and cell fate specification in the control of Caenorhabditis elegans gastrulation". Developmental Dynamics. 238 (4): 789–796. doi:10.1002/dvdy.21893. PMC 2929021 . PMID 19253398.
- ↑ Segalen M, Bellaiche Y: Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol 2009, 20:972-977. Segalen, M.; Bellaïche, Y. (2009). "Cell division orientation and planar cell polarity pathways". Seminars in Cell & Developmental Biology. 20 (8): 972–977. doi:10.1016/j.semcdb.2009.03.018. PMID 19447051.
- ↑ Fazi F, Nervi C: MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc Res 2008, 79:553-561. Fazi, F.; Nervi, C. (2008). "MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination". Cardiovascular Research. 79 (4): 553–561. doi: 10.1093/cvr/cvn151 . PMID 18539629.
- ↑ "Multiplex mode for the LSM 9 series with Airyscan 2: fast and gentle confocal super-resolution in large volumes" (PDF).
- ↑ Weissman, Tamily A.; Pan, Y. Albert (February 2015). "Brainbow: New Resources and Emerging Biological Applications for Multicolor Genetic Labeling and Analysis". Genetics. 199 (2): 293–306. doi:10.1534/genetics.114.172510. ISSN 0016-6731. PMC 4317644 . PMID 25657347.
- ↑ Friedmann-Morvinski, Dinorah; Verma, Inder M (March 2014). "Dedifferentiation and reprogramming: origins of cancer stem cells". EMBO Reports. 15 (3): 244–253. doi:10.1002/embr.201338254. ISSN 1469-221X. PMC 3989690 . PMID 24531722.
- ↑ Vibert, Laura; Daulny, Anne; Jarriault, Sophie (2018). "Wound healing, cellular regeneration and plasticity: the elegans way". The International Journal of Developmental Biology. 62 (6–7–8): 491–505. doi:10.1387/ijdb.180123sj. ISSN 0214-6282. PMC 6161810 . PMID 29938761.
- ↑ Shohayeb B, et al. (October 2021). "Conservation of neural progenitor identity and the emergence of neocortical neuronal diversity". Seminars in Cell and Developmental Biology. 118 (118): 4–13. doi:10.1016/j.semcdb.2021.05.024. PMID 34083116. S2CID 235336596.
- 1 2 3 4 5 Gilbert, Scott (2006). Developmental biology (8th ed.). Sunderland, Mass.: Sinauer Associates, Inc. Publishers. pp. 53–55. ISBN 978-0-87893-250-4.
- 1 2 3 4 5 Gilbert, S. F. (2000). Developmental Biology (6th ed.).
- ↑ Whittaker, JR (Jul 1973). "Segregation during ascidian embryogenesis of egg cytoplasmic information for tissue-specific enzyme development". PNAS. 70 (7): 2096–100. Bibcode:1973PNAS...70.2096W. doi: 10.1073/pnas.70.7.2096 . PMC 433673 . PMID 4198663.
- 1 2 Xiong, W.; Ferrell Jr, J. (2003). "A positive-feedback-based bistable 'memory module' that governs a cell fate decision". Nature. 426 (6965): 460–465. Bibcode:2003Natur.426..460X. doi:10.1038/nature02089. PMID 14647386. S2CID 4396489.
- 1 2 3 4 5 Gilbert, Scott (2014). Developmental Biology (10 ed.). Sinauer Associates, Inc.
- ↑ Guo G, Huss M, Tong GQ, Wang C, Li Sun L, Clarke ND, Robson P: Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst. Dev Cell 2010, 18:675-685.Guo, G.; Huss, M.; Tong, G.; Wang, C.; Li Sun, L.; Clarke, N.; Robson, P. (2010). "Resolution of cell fate decisions revealed by single-cell gene expression analysis from zygote to blastocyst". Developmental Cell. 18 (4): 675–685. doi: 10.1016/j.devcel.2010.02.012 . PMID 20412781.
- ↑ Cairns JM: Development of grafts from mouse embryos to the wing bud of the chick embryo. Dev Biol 1965, 12:36-52.Cairns, J. (1965). "Development of grafts from mouse embryos to the wing bud of the chick embryo". Developmental Biology. 12 (1): 36–00. doi:10.1016/0012-1606(65)90019-9. PMID 5833110.
- ↑ Nakamura, Taro; Yoshizaki, Masato; Ogawa, Shotaro; Okamoto, Haruko; Shinmyo, Yohei; Bando, Tetsuya; Ohuchi, Hideyo; Noji, Sumihare; Mito, Taro (2010-09-28). "Imaging of Transgenic Cricket Embryos Reveals Cell Movements Consistent with a Syncytial Patterning Mechanism". Current Biology. 20 (18): 1641–1647. doi: 10.1016/j.cub.2010.07.044 . ISSN 0960-9822. PMID 20800488. S2CID 11443065.
- ↑ Lau S, Ehrismann JS, Schlereth A, Takada S, Mayer U, Jurgens G: Cell-cell communication in Arabidopsis early embryogenesis. Eur J Cell Biol 2010, 89:225-230. Lau, S.; Ehrismann, J.; Schlereth, A.; Takada, S.; Mayer, U.; Jürgens, G. (2010). "Cell-cell communication in Arabidopsis early embryogenesis". European Journal of Cell Biology. 89 (2–3): 225–230. doi:10.1016/j.ejcb.2009.11.010. PMID 20031252.
- ↑ Briscoe, J (2009). "Making a grade: Sonic Hedgehog signalling and the control of neural cell fate". EMBO J. 28 (5): 457–465. doi:10.1038/emboj.2009.12. PMC 2647768 . PMID 19197245.
- ↑ Rabajante JF, Babierra AL (January 30, 2015). "Branching and oscillations in the epigenetic landscape of cell-fate determination". Progress in Biophysics and Molecular Biology. 117 (2–3): 240–249. doi:10.1016/j.pbiomolbio.2015.01.006. PMID 25641423. S2CID 2579314.