Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.
In organic chemistry, an alkyne trimerisation is a [2+2+2] cycloaddition reaction in which three alkyne units react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles.

Cavicularin is a natural phenolic secondary metabolite isolated from the liverwort Cavicularia densa. This macrocycle is unusual because it was the first compound isolated from nature displaying optical activity solely due to the presence of planar chirality and axial chirality. The specific rotation for (+)-cavicularin is +168.2°. It is also a very strained molecule. The para-substituted phenol ring is bent about 15° out of planarity, adopting a somewhat boat-like geometry. This type of angle strain in aromatic compounds is normally reserved for synthetic cyclophanes.

In organic chemistry, helicenes are ortho-condensed polycyclic aromatic compounds in which benzene rings or other aromatics are angularly annulated to give helically-shaped chiral molecules. The chemistry of helicenes has attracted continuing attention because of their unique structural, spectral, and optical features.
Pentazole is an aromatic molecule consisting of a five-membered ring with all nitrogen atoms, one of which is bonded to a hydrogen atom. It has the molecular formula HN5. Although strictly speaking a homocyclic, inorganic compound, pentazole has historically been classed as the last in a series of heterocyclic azole compounds containing one to five nitrogen atoms. This set contains pyrrole, imidazole, pyrazole, triazoles, tetrazole, and pentazole.
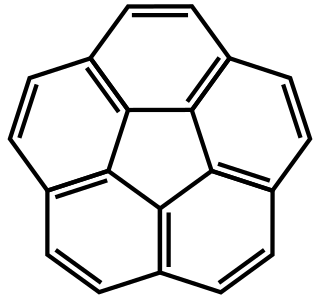
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.
Ring-closing metathesis (RCM) is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.

In organic chemistry, Bredt's rule is an empirical observation that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed it in 1902 and codified it in 1924. It primarily relates to bridgeheads with carbon-carbon and carbon-nitrogen double bonds.

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.
In organic chemistry, a cycloalkyne is the cyclic analog of an alkyne. A cycloalkyne consists of a closed ring of carbon atoms containing one or more triple bonds. Cycloalkynes have a general formula CnH2n−4. Because of the linear nature of the C−C≡C−C alkyne unit, cycloalkynes can be highly strained and can only exist when the number of carbon atoms in the ring is great enough to provide the flexibility necessary to accommodate this geometry. Large alkyne-containing carbocycles may be virtually unstrained, while the smallest constituents of this class of molecules may experience so much strain that they have yet to be observed experimentally. Cyclooctyne is the smallest cycloalkyne capable of being isolated and stored as a stable compound. Despite this, smaller cycloalkynes can be produced and trapped through reactions with other organic molecules or through complexation to transition metals.

Maitotoxin is an extremely powerful biotoxin produced by Gambierdiscus toxicus, a dinoflagellate species. Maitotoxin has been shown to be more than one hundred thousand times more potent than VX nerve agent. Maitotoxin is so potent that it has been demonstrated that an intraperitoneal injection of 130 ng/kg was lethal in mice. Maitotoxin was named from the ciguateric fish Ctenochaetus striatus—called "maito" in Tahiti—from which maitotoxin was isolated for the first time. It was later shown that maitotoxin is actually produced by the dinoflagellate Gambierdiscus toxicus.

In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene rings which have been linearly fused. They follow the general molecular formula C4n+2H2n+4.
[n]Radialenes are alicyclic organic compounds containing n cross-conjugated exocyclic double bonds. The double bonds are commonly alkene groups but those with a carbonyl (C=O) group are also called radialenes. For some members the unsubstituted parent radialenes are elusive but many substituted derivatives are known.

Roseophilin is an antibiotic isolated from Streptomyces griscovirides shown to have antitumor activity. The chemical structure can be considered in terms of two components, a macrotricyclic segment and a heterocyclic side-chain. Several laboratory syntheses of roseophilin are based upon the Paal-Knorr synthesis, and two others are based on the Nazarov cyclization reaction. The compound is related to the prodiginines.

Spirotryprostatin B is an indolic alkaloid found in the Aspergillus fumigatus fungus that belongs to a class of naturally occurring 2,5-diketopiperazines. Spirotryprostatin B and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.

PEPPSI is an abbreviation for pyridine-enhanced precatalyst preparation stabilization and initiation. It refers to a family of commercially available palladium catalysts developed around 2005 by Prof. Michael G. Organ and co-workers at York University, which can accelerate various carbon-carbon and carbon-heteroatom bond forming cross-coupling reactions. In comparison to many alternative palladium catalysts, Pd-PEPPSI-type complexes are stable to air and moisture and are relatively easy to synthesize and handle.
Münchnone (synonyms: 1,3-oxazolium-5-oxide; 1,3-oxazolium-5-olate; anhydro-5-hydroxy-1,3-oxazolium hydroxide; 5-hydroxy-1,3-oxazolium hydroxide, inner salt; oxido-oxazolium) is a mesoionic heterocyclic aromatic chemical compound, with the molecular formula C3H3NO2. The name refers to the city of Munich, Germany (German: München), where the compound and its derivatives were first discovered and studied.
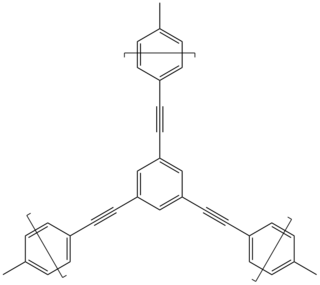
Conjugated microporous polymers (CMPs) are a sub-class of porous materials that are related to structures such as zeolites, metal-organic frameworks, and covalent organic frameworks, but are amorphous in nature, rather than crystalline. CMPs are also a sub-class of conjugated polymers and possess many of the same properties such as conductivity, mechanical rigidity, and insolubility. CMPs are created through the linking of building blocks in a π-conjugated fashion and possess 3-D networks. Conjugation extends through the system of CMPs and lends conductive properties to CMPs. Building blocks of CMPs are attractive in that the blocks possess broad diversity in the π units that can be used and allow for tuning and optimization of the skeleton and subsequently the properties of CMPs. Most building blocks have rigid components such as alkynes that cause the microporosity. CMPs have applications in gas storage, heterogeneous catalysis, light emitting, light harvesting, and electric energy storage.

In organic chemistry, the sulfonamide functional group is an organosulfur group with the structure R−S(=O)2−NR2. It consists of a sulfonyl group connected to an amine group. Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group.
In organic chemistry, hydrovinylation is the formal insertion of an alkene into the C-H bond of ethylene. The more general reaction, hydroalkenylation, is the formal insertion of an alkene into the C-H bond of any terminal alkene. The reaction is catalyzed by metal complexes. A representative reaction is the conversion of styrene and ethylene to 3-phenybutene:
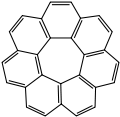
![[7]circulene -7-circulene.svg](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d4/-7-circulene.svg/110px--7-circulene.svg.png)