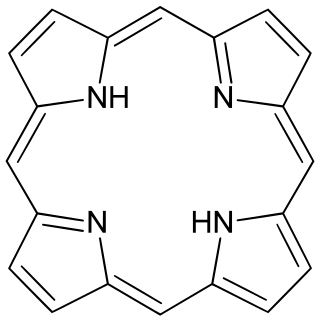
Porphyrins are a group of heterocyclic macrocycle organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−). In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream. In plants, an essential porphyrin derivative is chlorophyll, which is involved in light-harvesting and electron transfer in photosynthesis.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4n + 2 π electrons, where n is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4n + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time.

A silabenzene is a heteroaromatic compound containing one or more silicon atoms instead of carbon atoms in benzene. A single substitution gives silabenzene proper; additional substitutions give a disilabenzene, trisilabenzene, etc.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .

1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of its stoichiometric relationship to benzene, COT has been the subject of much research and some controversy.
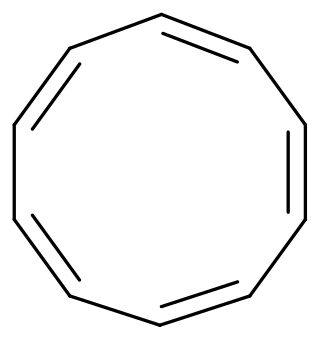
Cyclodecapentaene or [10]annulene is an annulene with molecular formula C10H10. This organic compound is a conjugated 10 pi electron cyclic system and according to Huckel's rule it should display aromaticity. It is not aromatic, however, because various types of ring strain destabilize an all-planar geometry.

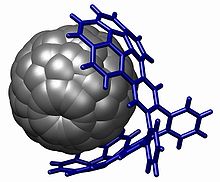
Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the molecule can be considered a fragment of buckminsterfullerene. Suman means "sunflower" in both Hindi and Sanskrit. The core of the arene is a benzene ring and the periphery consists of alternating benzene rings (3) and cyclopentadiene rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reactions.

Cyclooctadecanonaene or [18]annulene is an organic compound with chemical formula C
18H
18. It belongs to the class of highly conjugated compounds known as annulenes and is aromatic. The usual isomer that [18]annulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the cis,trans,trans,cis,trans,trans,cis,trans,trans configuration. It is reported to be a red-brown crystalline solid.

Annulynes or dehydroannulenes are conjugated monocyclic hydrocarbons with alternating single and double bonds in addition to at least one triple bond.

A geodesic polyarene in organic chemistry is a polycyclic aromatic hydrocarbon with curved convex or concave surfaces. Examples include fullerenes, nanotubes, corannulenes, helicenes and sumanene. The molecular orbitals of the carbon atoms in these systems are to some extent pyramidalized resulting a different pi electron density on either side of the molecule with consequences for reactivity.

Cyclododecahexaene or [12]annulene is a member of the series of annulenes with some interest in organic chemistry with regard to the study of aromaticity. Cyclododecahexaene is non-aromatic due to the lack of planarity of the structure. On the other hand the dianion with 14 electrons is a Hückel aromat and more stable.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
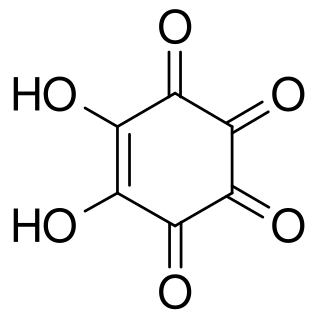
Rhodizonic acid is a chemical compound with formula C6H2O6 or (CO)4(COH)2. It can be seen as a twofold enol and fourfold ketone of cyclohexene, more precisely 5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone.

The Birch reduction is an organic reaction that is used to convert arenes to 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent with an alkali metal and a proton source. Unlike catalytic hydrogenation, Birch reduction does not reduce the aromatic ring all the way to a cyclohexane.
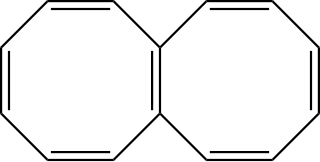
Octalene is a polycyclic hydrocarbon composed of two fused cyclooctatetraene rings.
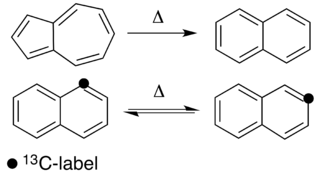
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.

Bis(cyclopentadienyl)titanium(III) chloride, also known as the Nugent–RajanBabu reagent, is the organotitanium compound which exists as a dimer with the formula [(C5H5)2TiCl]2. It is an air sensitive green solid. The complex finds specialized use in synthetic organic chemistry as a single electron reductant.

Contorted aromatics or more precisely contorted polycyclic aromatic hydrocarbons are polycyclic aromatic hydrocarbons (PAHs) in which the fused aromatic molecules deviate from the usual planarity.

Lithium naphthalene is an organic salt with the chemical formula Li+C
10H−
8. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+.



![Cyclopenta[bc]corannulene Cyclopenta-bc-corannulene.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/21/Cyclopenta-bc-corannulene.png/100px-Cyclopenta-bc-corannulene.png)