
Bacteriocins are proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacterial strain(s). They are similar to yeast and paramecium killing factors, and are structurally, functionally, and ecologically diverse. Applications of bacteriocins are being tested to assess their application as narrow-spectrum antibiotics.

Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell.

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Bordetella bronchiseptica and Bordetella parapertussis, also able to cause pertussis-like symptoms, also produce adenylate cyclase toxin. It is a toxin secreted by the bacteria to influence the host immune system.
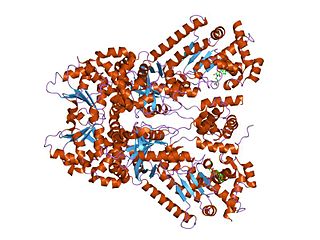
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis—the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen (PA), and two enzyme components, called edema factor (EF) and lethal factor (LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed. In the endosome, the enzymatic components of the toxin translocate into the cytoplasm of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupts various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage cells. Anthrax toxin allows the bacteria to evade the immune system, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight into the generation of macromolecular assemblies, and on protein translocation, pore formation, endocytosis, and other biochemical processes.
Cytolysin refers to the substance secreted by microorganisms, plants or animals that is specifically toxic to individual cells, in many cases causing their dissolution through lysis. Cytolysins that have a specific action for certain cells are named accordingly. For instance, the cytolysins responsible for the destruction of red blood cells, thereby liberating hemoglobins, are named hemolysins, and so on. Cytolysins may be involved in immunity as well as in venoms.
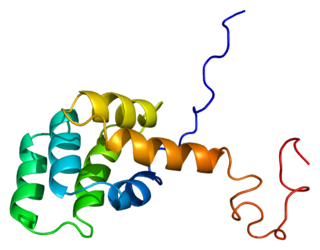
The Fas receptor, also known as Fas, FasR, apoptosis antigen 1, cluster of differentiation 95 (CD95) or tumor necrosis factor receptor superfamily member 6 (TNFRSF6), is a protein that in humans is encoded by the FAS gene. Fas was first identified using a monoclonal antibody generated by immunizing mice with the FS-7 cell line. Thus, the name Fas is derived from FS-7-associated surface antigen.
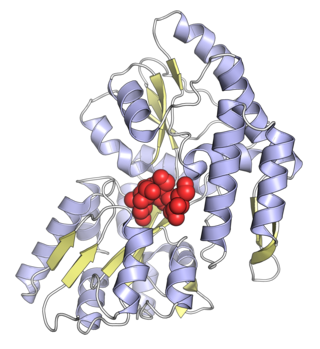
Maltose-binding protein (MBP) is a part of the maltose/maltodextrin system of Escherichia coli, which is responsible for the uptake and efficient catabolism of maltodextrins. It is a complex regulatory and transport system involving many proteins and protein complexes. MBP has an approximate molecular mass of 42.5 kilodaltons.

Hemolysins or haemolysins are lipids and proteins that cause lysis of red blood cells by disrupting the cell membrane. Although the lytic activity of some microbe-derived hemolysins on red blood cells may be of great importance for nutrient acquisition, many hemolysins produced by pathogens do not cause significant destruction of red blood cells during infection. However, hemolysins are often capable of lysing red blood cells in vitro.

Outer membrane receptors, also known as TonB-dependent receptors, are a family of beta barrel proteins named for their localization in the outer membrane of gram-negative bacteria. TonB complexes sense signals from the outside of bacterial cells and transmit them into the cytoplasm, leading to transcriptional activation of target genes. TonB-dependent receptors in gram-negative bacteria are associated with the uptake and transport of large substrates such as iron siderophore complexes and vitamin B12.

Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.
Streptolysins are two hemolytic exotoxins from Streptococcus. Types include streptolysin O, which is oxygen-labile, and streptolysin S, which is oxygen-stable.
'Staphylococcus aureus delta toxin is a toxin produced by Staphylococcus aureus. It has a wide spectrum of cytolytic activity.
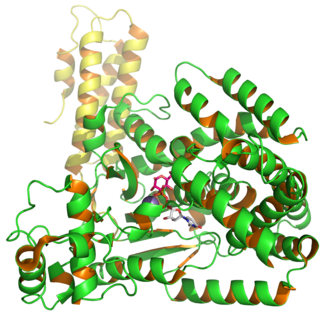
Clostridium difficile toxin B is a cytotoxin produced by the bacteria Clostridioides difficile, formerly known as Clostridium difficile. It is one of two major kinds of toxins produced by C. difficile, the other being an related enterotoxin. Both are very potent and lethal.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.
The CTXφ bacteriophage is a filamentous bacteriophage. It is a positive-strand DNA virus with single-stranded DNA (ssDNA).
Many bacteria secrete small iron-binding molecules called siderophores, which bind strongly to ferric ions. FepA is an integral bacterial outer membrane porin protein that belongs to outer membrane receptor family and provides the active transport of iron bound by the siderophore enterobactin from the extracellular space, into the periplasm of Gram-negative bacteria. FepA has also been shown to transport vitamin B12, and colicins B and D as well. This protein belongs to family of ligand-gated protein channels.

Bacteriocin AS-48 is a cyclic peptide antibiotic produced by the eubacteria Enterococcus faecalis that shows a broad antimicrobial spectrum against both Gram-positive and Gram-negative bacteria. Bacteriocin AS-48 is encoded by the pheromone-responsive plasmid pMB2, and acts on the plasma membrane in which it opens pores leading to ion leakage and cell death. The globular structure of bacteriocin AS-48 is composed of five alpha helices enclosing a hydrophobic core. The mammalian NK-lysin effector protein of T and natural killer cells has a similar structure, though it lacks sequence homology with bacteriocins AS-48.

Gasdermin D (GSDMD) is a protein that in humans is encoded by the GSDMD gene on chromosome 8. It belongs to the gasdermin family which is conserved among vertebrates and comprises six members in humans, GSDMA, GSDMB, GSDMC, GSDMD, GSDME (DFNA5) and DFNB59 (Pejvakin). Members of the gasdermin family are expressed in a variety of cell types including epithelial cells and immune cells. GSDMA, GSDMB, GSDMC, GSDMD and GSDME have been suggested to act as tumour suppressors.
Pyocins are bacteriocins produced by bacteria belonging to the Pseudomonas genus. François Jacob described the first pyocin in 1954. Pyocins can be divided into three distinct classes: S-type, R-type, and F-type pyocins. S-type pyocins are colicin-like bacteriocins as R-type and F-type pyocins belong to tailocins.












