Related Research Articles

Potassium is a chemical element; it has symbol K and atomic number 19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge. In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in seawater, and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.

Salinity is the saltiness or amount of salt dissolved in a body of water, called saline water. It is usually measured in g/L or g/kg.
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.

Clinical chemistry is a division in medical laboratory sciences focusing on qualitative tests of important compounds, referred to as analytes or markers, in bodily fluids and tissues using analytical techniques and specialized instruments. This interdisciplinary field includes knowledge from medicine, biology, chemistry, biomedical engineering, informatics, and an applied form of biochemistry.
The term chloride refers either to a chloride ion, which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is.

Brine is a high-concentration solution of salt in water. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% up to about 26%. Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking, for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization.

Sodium chloride, commonly known as table salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.

Potassium chloride is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt-like taste. Potassium chloride can be obtained from ancient dried lake deposits. KCl is used as a fertilizer, in medicine, in scientific applications, domestic water softeners, and in food processing, where it may be known as E number additive E508.
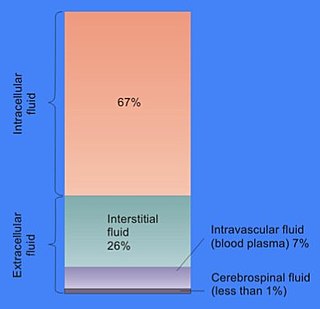
In cell biology, extracellular fluid (ECF) denotes all body fluid outside the cells of any multicellular organism. Total body water in healthy adults is about 50–60% of total body weight; women and the obese typically have a lower percentage than lean men. Extracellular fluid makes up about one-third of body fluid, the remaining two-thirds is intracellular fluid within cells. The main component of the extracellular fluid is the interstitial fluid that surrounds cells.

Renal physiology is the study of the physiology of the kidney. This encompasses all functions of the kidney, including maintenance of acid-base balance; regulation of fluid balance; regulation of sodium, potassium, and other electrolytes; clearance of toxins; absorption of glucose, amino acids, and other small molecules; regulation of blood pressure; production of various hormones, such as erythropoietin; and activation of vitamin D.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include calcium, chloride, magnesium, phosphate, potassium, and sodium.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, it is used to treat dehydration such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.

Ringer's lactate solution (RL), also known as sodium lactate solution,Lactated Ringer's, and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area.

Saline water is water that contains a high concentration of dissolved salts. On the United States Geological Survey (USGS) salinity scale, saline water is saltier than brackish water, but less salty than brine. The salt concentration is usually expressed in parts per thousand and parts per million (ppm). The USGS salinity scale defines three levels of saline water. The salt concentration in slightly saline water is 1,000 to 3,000 ppm (0.1–0.3%); in moderately saline water is 3,000 to 10,000 ppm (0.3–1%); and in highly saline water is 10,000 to 35,000 ppm (1–3.5%). Seawater has a salinity of roughly 35,000 ppm, equivalent to 35 grams of salt per one liter of water. The saturation level is only nominally dependent on the temperature of the water. At 20 °C (68 °F) one liter of water can dissolve about 357 grams of salt, a concentration of 26.3 percent by weight. At 100 °C (212 °F), the amount of salt that can be dissolved in one liter of water increases to about 391 grams, a concentration of 28.1% w/w.
The Na–K–Cl cotransporter (NKCC) is a transport protein that aids in the secondary active transport of sodium, potassium, and chloride into cells. In humans there are two isoforms of this membrane transport protein, NKCC1 and NKCC2, encoded by two different genes. Two isoforms of the NKCC1/Slc12a2 gene result from keeping or skipping exon 21 in the final gene product.
A volume expander is a type of intravenous therapy that has the function of providing volume for the circulatory system. It may be used for fluid replacement or during surgery to prevent nausea and vomiting after surgery.

Conductivity of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter (S/m).

Ringer's solution is a solution of several salts dissolved in water for the purpose of creating an isotonic solution relative to the body fluids of an animal. Ringer's solution typically contains sodium chloride, potassium chloride, calcium chloride and sodium bicarbonate, with the last used to balance the pH. Other additions can include chemical fuel sources for cells, including ATP and dextrose, as well as antibiotics and antifungals.
A balanced salt solution (BSS) is a solution made to a physiological pH and isotonic salt concentration. Solutions most commonly include sodium, potassium, calcium, magnesium, and chloride. Balanced salt solutions are used for washing tissues and cells and are usually combined with other agents to treat the tissues and cells. They provide the cells with water and inorganic ions, while maintaining a physiological pH and osmotic pressure.
References
- ↑ Weast, Robert C. (1982). CRC Handbook of Chemistry and Physics: A Ready-reference Book of Chemical and Physical Data. CRC Press. p. 2082. ISBN 9780849304637.
- ↑ Wolf, Arnold Veryl (1966). Aqueous solutions and body fluids: their concentrative properties and conversion tables. Hoeber Medical Division, Harper & Row. pp. 17–28.
- 1 2 A Slomowitz, Larry; Deng, Aihua; S Hammes, John; Gabbai, Francis; C Thomson, Scott (2002-05-01). "Glomerulotubular balance, dietary protein, and the renal response to glycine in diabetic rats". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 282 (4): R1096–103. doi:10.1152/ajpregu.00610.2001. PMID 11893614. S2CID 13630228.
- ↑ Hume, Ian D. (1999-05-27). Marsupial Nutrition. Cambridge University Press. p. 289. ISBN 9780521595551.
- ↑ N.Y.), Society for Experimental Biology and Medicine (New York (1967). Proceedings of the Society for Experimental Biology and Medicine. p. 938.
- ↑ Marañon, T.; García, L. V.; Troncoso, A. (1989-10-01). "Salinity and germination of annual Melilotus from the Guadalquivir delta (SW Spain)". Plant and Soil. 119 (2): 223–228. doi:10.1007/BF02370412. hdl: 10261/11766 . ISSN 0032-079X. S2CID 39027165.
- ↑ Wolf, A.V.; Pillay, V.K.G (June 1969). "Renal Concentration Tests: Osmotic Pressure, Specific Gravity, Refraction and Electrical Conductivity Compared". The American Journal of Medicine. 46 (6): 838–839. doi:10.1016/0002-9343(69)90085-0. PMID 5797912 . Retrieved 1 January 2018.
- ↑ Wolf, Arnold Veryl (1966). "Electrical Conductivity: Specific Conductance, Condosity and Relative Salinity". Aqueous solutions and body fluids: their concentrative properties and conversion tables. New York: Hoeber Medical Division, Harper & Row. pp. 19–26.