Potassium is a chemical element; it has symbol K and atomic number 19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge. In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in seawater, and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.

Sodium is a chemical element; it has symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans.

Potassium ferrocyanide is the inorganic compound with formula K4[Fe(CN)6]·3H2O. It is the potassium salt of the coordination complex [Fe(CN)6]4−. This salt forms lemon-yellow monoclinic crystals.

Potash includes various mined and manufactured salts that contain potassium in water-soluble form. The name derives from pot ash, plant ashes or wood ash soaked in water in a pot, the primary means of manufacturing potash before the Industrial Era. The word potassium is derived from potash.
The term chloride refers either to a chloride ion, which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is.

Sodium chloride, commonly known as table salt, is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, it is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is deicing of roadways in sub-freezing weather.

Sodium hypochlorite is an alkaline inorganic chemical compound with the formula NaOCl. It is commonly known in a dilute aqueous solution as bleach or chlorine bleach. It is the sodium salt of hypochlorous acid, consisting of sodium cations and hypochlorite anions.

Potassium hydroxide is an inorganic compound with the formula KOH, and is commonly called caustic potash.

In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.

Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.

Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K2SO4, a white water-soluble solid. It is commonly used in fertilizers, providing both potassium and sulfur.
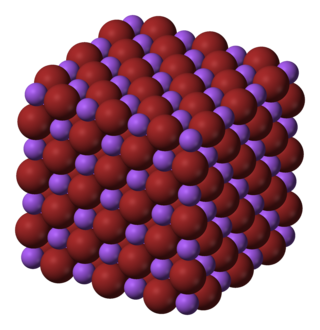
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.

Carnallite (also carnalite) is an evaporite mineral, a hydrated potassium magnesium chloride with formula KCl.MgCl2·6(H2O). It is variably colored yellow to white, reddish, and sometimes colorless or blue. It is usually massive to fibrous with rare pseudohexagonal orthorhombic crystals. The mineral is deliquescent (absorbs moisture from the surrounding air) and specimens must be stored in an airtight container.

A salt substitute, also known as low-sodium salt, is a low-sodium alternative to edible salt marketed to reduce the risk of high blood pressure and cardiovascular disease associated with a high intake of sodium chloride while maintaining a similar taste.
A balanced salt solution (BSS) is a solution made to a physiological pH and isotonic salt concentration. Solutions most commonly include sodium, potassium, calcium, magnesium, and chloride. Balanced salt solutions are used for washing tissues and cells and are usually combined with other agents to treat the tissues and cells. They provide the cells with water and inorganic ions, while maintaining a physiological pH and osmotic pressure.
Potassium hypochlorite is a chemical compound with the chemical formula KOCl, also written as KClO. It is the potassium salt of hypochlorous acid. It consists of potassium cations and hypochlorite anions. It is used in variable concentrations, often diluted in water solution. Its aqueous solutions are colorless liquids that have a strong chlorine smell. It is used as a biocide and disinfectant.

Leonite is a hydrated double sulfate of magnesium and potassium. It has the formula K2SO4·MgSO4·4H2O. The mineral was named after Leo Strippelmann, who was director of the salt works at Westeregeln in Germany. The mineral is part of the blodite group of hydrated double sulfate minerals.

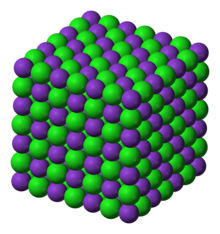
Potassium chloride, also known as potassium salt, is used as a medication to treat and prevent low blood potassium. Low blood potassium may occur due to vomiting, diarrhea, or certain medications. The concentrated version should be diluted before use. It is given by slow injection into a vein or by mouth.