
Carbon dioxide is a chemical compound with the chemical formula CO2. It is made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature, and as the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. When carbon dioxide dissolves in water, it forms carbonate and mainly bicarbonate, which causes ocean acidification as atmospheric CO2 levels increase.

Carbon monoxide is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry.

Sulfur dioxide or sulphur dioxide is the chemical compound with the formula SO
2. It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur-bearing fossil fuels. It was known to alchemists as "volatile spirit of sulfur" since at least 16th century.

Hydrogen sulfide is a chemical compound with the formula H2S. It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.

Cloud condensation nuclei (CCNs), also known as cloud seeds, are small particles typically 0.2 µm, or one hundredth the size of a cloud droplet. CCNs are a unique subset of aerosols in the atmosphere on which water vapour condenses. This can affect the radiative properties of clouds and the overall atmosphere. Water vapour requires a non-gaseous surface to make the transition to a liquid; this process is called condensation.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.

Sulfuryl fluoride (also spelled sulphuryl fluoride) is an inorganic compound with the formula SO2F2. It is an easily condensed gas and has properties more similar to sulfur hexafluoride than sulfuryl chloride, being resistant to hydrolysis even up to 150 °C. It is neurotoxic and a potent greenhouse gas, but is widely used as a fumigant insecticide to control termites.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.

The infrared atmospheric window refers to a region of the infrared spectrum where there is relatively little absorption of terrestrial thermal radiation by atmospheric gases. The window plays an important role in the atmospheric greenhouse effect by maintaining the balance between incoming solar radiation and outgoing IR to space. In the Earth's atmosphere this window is roughly the region between 8 and 14 μm although it can be narrowed or closed at times and places of high humidity because of the strong absorption in the water vapor continuum or because of blocking by clouds. It covers a substantial part of the spectrum from surface thermal emission which starts at roughly 5 μm. Principally it is a large gap in the absorption spectrum of water vapor. Carbon dioxide plays an important role in setting the boundary at the long wavelength end. Ozone partly blocks transmission in the middle of the window.
Mass-independent isotope fractionation or Non-mass-dependent fractionation (NMD), refers to any chemical or physical process that acts to separate isotopes, where the amount of separation does not scale in proportion with the difference in the masses of the isotopes. Most isotopic fractionations are caused by the effects of the mass of an isotope on atomic or molecular velocities, diffusivities or bond strengths. Mass-independent fractionation processes are less common, occurring mainly in photochemical and spin-forbidden reactions. Observation of mass-independently fractionated materials can therefore be used to trace these types of reactions in nature and in laboratory experiments.

Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. The simplest thioether, it is a flammable liquid that boils at 37 °C (99 °F) and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol.

Tetrafluoromethane, also known as carbon tetrafluoride or R-14, is the simplest perfluorocarbon (CF4). As its IUPAC name indicates, tetrafluoromethane is the perfluorinated counterpart to the hydrocarbon methane. It can also be classified as a haloalkane or halomethane. Tetrafluoromethane is a useful refrigerant but also a potent greenhouse gas. It has a very high bond strength due to the nature of the carbon–fluorine bond.

Károly Antal Than de Apát – also called as Carl von Than – was a Hungarian chemist who discovered carbonyl sulfide in 1867.
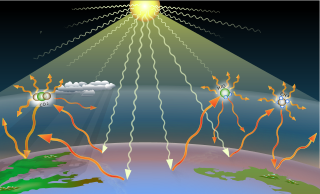
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. What distinguishes them from other gases is that they absorb the wavelengths of radiation that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about −18 °C (0 °F), rather than the present average of 15 °C (59 °F).

Stratospheric aerosol injection is a proposed method of solar geoengineering to reduce global warming. This would introduce aerosols into the stratosphere to create a cooling effect via global dimming and increased albedo, which occurs naturally from volcanic winter. It appears that stratospheric aerosol injection, at a moderate intensity, could counter most changes to temperature and precipitation, take effect rapidly, have low direct implementation costs, and be reversible in its direct climatic effects. The Intergovernmental Panel on Climate Change concludes that it "is the most-researched [solar geoengineering] methodagreement that it could limit warming to below 1.5 °C (2.7 °F)." However, like other solar geoengineering approaches, stratospheric aerosol injection would do so imperfectly and other effects are possible, particularly if used in a suboptimal manner.
Tectonic–climatic interaction is the interrelationship between tectonic processes and the climate system. The tectonic processes in question include orogenesis, volcanism, and erosion, while relevant climatic processes include atmospheric circulation, orographic lift, monsoon circulation and the rain shadow effect. As the geological record of past climate changes over millions of years is sparse and poorly resolved, many questions remain unresolved regarding the nature of tectonic-climate interaction, although it is an area of active research by geologists and palaeoclimatologists.
Sulfanyl (HS•), also known as the mercapto radical, hydrosulfide radical, or hydridosulfur, is a simple radical molecule consisting of one hydrogen and one sulfur atom. The radical appears in metabolism in organisms as H2S is detoxified. Sulfanyl is one of the top three sulfur-containing gasses in gas giants such as Jupiter and is very likely to be found in brown dwarfs and cool stars. It was originally discovered by Margaret N. Lewis and John U. White at the University of California in 1939. They observed molecular absorption bands around 325 nm belonging to the system designated by 2Σ+ ← 2Πi. They generated the radical by means of a radio frequency discharge in hydrogen sulfide. HS• is formed during the degradation of hydrogen sulfide in the atmosphere of the Earth. This may be a deliberate action to destroy odours or a natural phenomenon.

Disulfur dioxide, dimeric sulfur monoxide or SO dimer is an oxide of sulfur with the formula S2O2. The solid is unstable with a lifetime of a few seconds at room temperature.
Carbonyl sulfide hydrolase (EC 3.13.1.7; abbreviated as COSase) is an enzyme that degrades carbonyl sulfide (COS) to hydrogen sulfide (H2S) and carbon dioxide (CO2). Isolated from Thiobacillus thioparus bacterium, the potential of COSase would reduce the high global warming effect of COS and change the ozone chemistry, because COS is the source of sulfur in the troposphere.






















