Aluminium is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating.

Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group.

Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray solid which shares many physical and chemical properties with the other five alkaline earth metals.

Scandium is a chemical element with the symbol Sc and atomic number 21.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Aluminium nitride (AlN) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potential application in optoelectronics operating at deep ultraviolet frequencies.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
Neodymium(III) chloride or neodymium trichloride is a chemical compound of neodymium and chlorine with the formula NdCl3. This anhydrous compound is a mauve-colored solid that rapidly absorbs water on exposure to air to form a purple-colored hexahydrate, NdCl3·6H2O. Neodymium(III) chloride is produced from minerals monazite and bastnäsite using a complex multistage extraction process. The chloride has several important applications as an intermediate chemical for production of neodymium metal and neodymium-based lasers and optical fibers. Other applications include a catalyst in organic synthesis and in decomposition of waste water contamination, corrosion protection of aluminium and its alloys, and fluorescent labeling of organic molecules (DNA).

Aluminium chloride (AlCl3), also known as aluminium trichloride, describe compounds with the formula AlCl3(H2O)n (n = 0 or 6). They consist of aluminium and chlorine atoms in a 1:3 ratio, and one form also contains six waters of hydration. Both are white solids, but samples are often contaminated with iron(III) chloride, giving a yellow color.
A superatom is any cluster of atoms that seem to exhibit some of the properties of elemental atoms.

Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a catalyst.
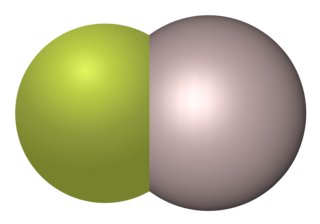
Aluminium monofluoride, also known as fluoridoaluminium, is the chemical compound with the formula AlF. This elusive species is formed by the reaction between aluminium trifluoride and metallic aluminium at elevated temperatures but quickly reverts to the reactants when cooled. Clusters derived from related aluminium(I) halides can be stabilized using specialized ligands.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water.

Aluminium hydride (also known as alane or alumane) is an inorganic compound with the formula AlH3. It presents as a white solid and may be tinted grey with decreasing particle size and impurity levels. Depending upon synthesis conditions, the surface of the alane may be passivated with a thin layer of aluminum oxide and/or hydroxide. Alane and its derivatives are used as reducing agents in organic synthesis.

Aluminium borohydride, also known as aluminium tetrahydroborate, (in American English, aluminum borohydride and aluminum tetrahydroborate, respectively) is the chemical compound with the formula Al(BH4)3. It is a volatile pyrophoric liquid which is used as a reducing agent in laboratories. Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound.

Zirconium(III) chloride is an inorganic compound with formula ZrCl3. It is a blue-black solid that is highly sensitive to air.
Indium(III) hydroxide is the chemical compound with the formula In(OH)3. Its prime use is as a precursor to indium(III) oxide, In2O3. It is sometimes found as the rare mineral dzhalindite.
In chemistry, aluminium(I) refers to monovalent aluminium in both ionic and covalent bonds. Along with aluminium(II), it is an extremely unstable form of aluminium.

(Pentamethylcyclopentadienyl)aluminium(I) is an organometallic compound with the formula Al(C5Me5) ("Me" is a methyl group; CH3). The compound is often abbreviated to AlCp* or Cp*Al, where Cp* is the pentamethylcyclopentadienide anion (C5Me5−). Discovered in 1991 by Dhmeier et al., AlCp* serves as the first ever documented example of a room temperature stable monovalent aluminium compound. In its isolated form, Cp*Al exists as the tetramer [Cp*Al]4, and is a yellow crystal that decomposes at temperatures above 100 °C but also sublimes at temperatures above 140 °C.

Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.