Photoconductivity is an optical and electrical phenomenon in which a material becomes more electrically conductive due to the absorption of electromagnetic radiation such as visible light, ultraviolet light, infrared light, or gamma radiation.
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow salt. It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century.

Sulfur monoxide is an inorganic compound with formula SO. It is only found as a dilute gas phase. When concentrated or condensed, it converts to S2O2 (disulfur dioxide). It has been detected in space but is rarely encountered intact otherwise.
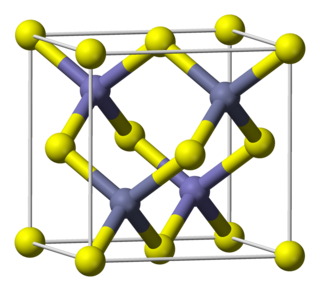
Indium antimonide (InSb) is a crystalline compound made from the elements indium (In) and antimony (Sb). It is a narrow-gap semiconductor material from the III-V group used in infrared detectors, including thermal imaging cameras, FLIR systems, infrared homing missile guidance systems, and in infrared astronomy. Indium antimonide detectors are sensitive to infrared wavelengths between 1 and 5 μm.

Mercury sulfide, or mercury(II) sulfide is a chemical compound composed of the chemical elements mercury and sulfur. It is represented by the chemical formula HgS. It is virtually insoluble in water.

Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment, but applications are declining because of environmental concerns.

Magnesium sulfide is an inorganic compound with the formula MgS. It is a white crystalline material but often is encountered in an impure form that is brown and non-crystalline powder. It is generated industrially in the production of metallic iron.

Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.

An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).

Platinum silicide, also known as platinum monosilicide, is the inorganic compound with the formula PtSi. It is a semiconductor that turns into a superconductor when cooled to 0.8 K.

Indium arsenide, InAs, or indium monoarsenide, is a narrow-bandgap semiconductor composed of indium and arsenic. It has the appearance of grey cubic crystals with a melting point of 942 °C.
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide.
Indium(III) sulfide (Indium sesquisulfide, Indium sulfide (2:3), Indium (3+) sulfide) is the inorganic compound with the formula In2S3.

A quantum dot solar cell (QDSC) is a solar cell design that uses quantum dots as the captivating photovoltaic material. It attempts to replace bulk materials such as silicon, copper indium gallium selenide (CIGS) or cadmium telluride (CdTe). Quantum dots have bandgaps that are adjustable across a wide range of energy levels by changing their size. In bulk materials, the bandgap is fixed by the choice of material(s). This property makes quantum dots attractive for multi-junction solar cells, where a variety of materials are used to improve efficiency by harvesting multiple portions of the solar spectrum.
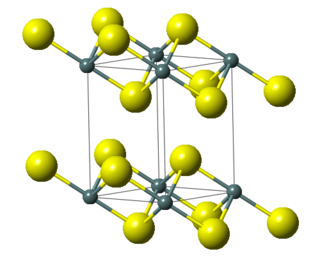
Lead(IV) sulfide is a chemical compound with the formula PbS2. This material is generated by the reaction of the more common lead(II) sulfide, PbS, with sulfur at >600 °C and at high pressures. PbS2, like the related tin(IV) sulfide SnS2, crystallises in the cadmium iodide motif, which indicates that Pb should be assigned the formal oxidation state of 4+.
A gas detector is a device that detects the presence of gases in an area, often as part of a safety system. A gas detector can sound an alarm to operators in the area where the leak is occurring, giving them the opportunity to leave. This type of device is important because there are many gases that can be harmful to organic life, such as humans or animals.
Quantum dots (QDs) are semiconductor nanoparticles with a size less than 10 nm. They exhibited size-dependent properties especially in the optical absorption and the photoluminescence (PL). Typically, the fluorescence emission peak of the QDs can be tuned by changing their diameters. So far, QDs were consisted of different group elements such as CdTe, CdSe, CdS in the II-VI category, InP or InAs in the III-V category, CuInS2 or AgInS2 in the I–III–VI2 category, and PbSe/PbS in the IV-VI category. These QDs are promising candidates as fluorescent labels in various biological applications such as bioimaging, biosensing and drug delivery.




















