
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.
In chemistry, titanate usually refers to inorganic compounds composed of titanium oxides, or oxides containing the titanium element. Together with niobate, titanate salts form the Perovskite group.

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.
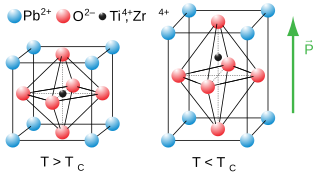
Lead zirconate titanate, also called lead zirconium titanate and commonly abbreviated as PZT, is an inorganic compound with the chemical formula Pb[ZrxTi1−x]O3(0 ≤ x ≤ 1). It is a ceramic perovskite material that shows a marked piezoelectric effect, meaning that the compound changes shape when an electric field is applied. It is used in a number of practical applications such as ultrasonic transducers and piezoelectric resonators. It is a white to off-white solid.
Multiferroics are defined as materials that exhibit more than one of the primary ferroic properties in the same phase:

Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics.
Bismuth ferrite (BiFeO3, also commonly referred to as BFO in materials science) is an inorganic chemical compound with perovskite structure and one of the most promising multiferroic materials. The room-temperature phase of BiFeO3 is classed as rhombohedral belonging to the space group R3c. It is synthesized in bulk and thin film form and both its antiferromagnetic (G type ordering) Néel temperature (approximately 653 K) and ferroelectric Curie temperature are well above room temperature (approximately 1100K). Ferroelectric polarization occurs along the pseudocubic direction () with a magnitude of 90–95 μC/cm2.
Lead scandium tantalate (PST) is a mixed oxide of lead, scandium, and tantalum. It has the formula Pb(Sc0.5Ta0.5)O3. It is a ceramic material with a perovskite structure, where the Sc and Ta atoms at the B site have an arrangement that is intermediate between ordered and disordered configurations, and can be fine-tuned with thermal treatment. It is ferroelectric at temperatures below 270 K (−3 °C; 26 °F), and is also piezoelectric. Like structurally similar lead zirconate titanate and barium strontium titanate, PST can be used for manufacture of uncooled focal plane array infrared imaging sensors for thermal cameras.
The Burns temperature, Td, is the temperature where a ferroelectric material, previously in paraelectric state, starts to present randomly polarized nanoregions, that are polar precursor clusters. This behaviour is typical of several, but not all, ferroelectric materials, and was observed in lead titanate (PbTiO3), potassium niobate (KNbO3), lead lanthanum zirconate titanate (PLZT), lead magnesium niobate (PMN), lead zinc niobate (PZN), K2Sr4(NbO3)10, and strontium barium niobate (SBN), Na1/2Bi1/2O3 (NBT).

Lithium titanates are chemical compounds of lithium, titanium and oxygen. They are mixed oxides and belong to the titanates. The most important lithium titanates are:

Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula CaTiO3). Its name is also applied to the class of compounds which have the same type of crystal structure as CaTiO3, known as the perovskite structure, which has a general chemical formula A2+B4+(X2−)3. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials.

Calcium titanate is an inorganic compound with the chemical formula CaTiO3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities.

Changbaiite (PbNb2O6) is a member of the oxide mineral class in which the mineral contains oxygen which is grouped along with one or two metal ion. Changbaiite is classified as a multiple Oxide XY2O6 and it generally has an ionic bond. Furthermore, it is also orthorhombic at a temperature of 25 °C and it changes to orthorhombic-tetragonal at 570 °C.

Quantum paraelectricity is a type of incipient ferroelectricity where the onset of ferroelectric order is suppressed by quantum fluctuations. From the soft mode theory of ferroelectricity, this occurs when a ferroelectric instability is stabilized by quantum fluctuations. In this case the soft-mode frequency never becomes unstable as opposed to a regular ferroelectric.
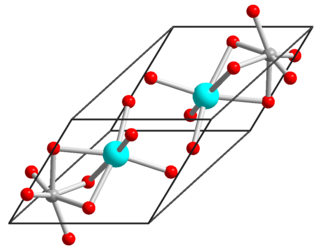
Nickel(II) titanate, also known as nickel titanium oxide, is an inorganic compound with the chemical formula NiTiO3. It is a coordination compound between nickel(II), titanium(IV) and oxide ions. It has the appearance of a yellow powder. Nickel(II) titanate has been used as a catalyst for toluene oxidation.
Sodium bismuth titanate or bismuth sodium titanium oxide (NBT or BNT) is a solid inorganic compound of sodium, bismuth, titanium and oxygen with the chemical formula of Na0.5Bi0.5TiO3 or Bi0.5Na0.5TiO3. This compound adopts the perovskite structure.
Macedonite is a mineral named by Radusinović and Markov in 1971. It has the elemental formula PbTiO3 and exhibits tetragonal crystal system. The type locality is near Crni Kamen, Selecka Planina, Prilep Municipality, North Macedonia. It can be confused with perovskite. It is found in an amazonite-rich area.

Europium(II) titanate is a black mixed oxide of europium and titanium, with the chemical formula of EuTiO3. It crystallizes in the perovskite structure.



 [1]
[1] 











