
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Ductility refers to the ability of a material to sustain significant plastic deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic deformation, which is reversible upon removing the stress. Ductility is a critical mechanical performance indicator, particularly in applications that require materials to bend, stretch, or deform in other ways without breaking. The extent of ductility can be quantitatively assessed using the percent elongation at break, given by the equation:

The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.

Nonmetals are chemical elements that mostly lack distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter than metals; brittle or crumbly if solid; and often poor conductors of heat and electricity. Chemically, nonmetals have high electronegativity ; and their oxides tend to be acidic.

A noble metal is ordinarily regarded as a metallic chemical element that is generally resistant to corrosion and is usually found in nature in its raw form. Gold, platinum, and the other platinum group metals are most often so classified. Silver, copper, and mercury are sometimes included as noble metals, but each of these usually occurs in nature combined with sulfur.
Nickel hydride is either an inorganic compound of the formula NiHx or any of a variety of coordination complexes. It was discovered by Polish chemist Bogdan Baranowski in 1958.
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

In mineralogy, argentite (from Latin argentum ' silver') is cubic silver sulfide (Ag2S), which can only exist at temperatures above 173 °C (343 °F), 177 °C (351 °F), or 179 °C (354 °F). When it cools to ordinary temperatures it turns into its monoclinic polymorph, acanthite. The International Mineralogical Association has decided to reject argentite as a proper mineral.

Zinc sulfide is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics.

Acanthite is a form of silver sulfide with the chemical formula Ag2S. It crystallizes in the monoclinic system and is the stable form of silver sulfide below 173 °C (343 °F). Argentite is the stable form above that temperature. As argentite cools below that temperature its cubic form is distorted to the monoclinic form of acanthite. Below 173 °C acanthite forms directly. Acanthite is the only stable form in normal air temperature.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This vivid orange, opaque crystalline compound is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.
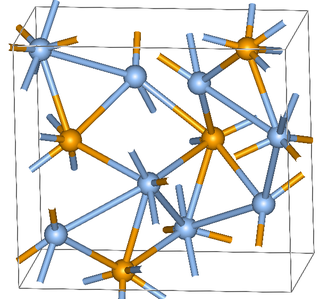
Silver selenide (Ag2Se) is the reaction product formed when selenium toning analog silver gelatine photo papers in photographic print toning. The selenium toner contains sodium selenite (Na2SeO3) as one of its active ingredients, which is the source of the selenide (Se2−) anion combining with the silver in the toning process.
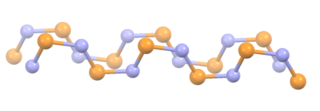
Polythiazyl, (SN)x, is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered and was also found to be a superconductor at very low temperatures. It is a fibrous solid, described as "lustrous golden on the faces and dark blue-black", depending on the orientation of the sample. It is air stable and insoluble in all solvents.
Indium(III) sulfide (Indium sesquisulfide, Indium sulfide (2:3), Indium (3+) sulfide) is the inorganic compound with the formula In2S3.

Aguilarite is an uncommon sulfosalt mineral with formula Ag4SeS. It was described in 1891 and named for discoverer Ponciano Aguilar.

Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.
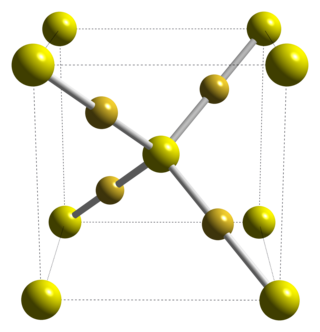
Gold(I) sulfide is the inorganic compound with the formula Au2S. It is the principal sulfide of gold. It decomposes to gold metal and elemental sulfur, illustrating the "nobility" of gold.

Gallium(III) sulfide, Ga2S3, is a compound of sulfur and gallium, that is a semiconductor that has applications in electronics and photonics.
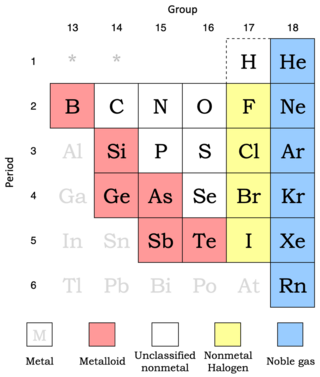
Nonmetals show more variability in their properties than do metals. Metalloids are included here since they behave predominately as chemically weak nonmetals.


 [2]
[2] 















