
Nucleic acids are large biomolecules that are crucial in all cells and viruses. They are composed of nucleotides, which are the monomer components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main classes of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). If the sugar is ribose, the polymer is RNA; if the sugar is deoxyribose, a variant of ribose, the polymer is DNA.

Johannes Friedrich Miescher was a Swiss physician and biologist. He was the first scientist to isolate nucleic acid in 1869. He also identified protamine and made several other discoveries.

Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Heparin is a blood anticoagulant that increases the activity of antithrombin. It is used in the treatment of heart attacks and unstable angina. It can be given intravenously or by injection under the skin. Its anticoagulant properties make it useful to prevent blood clotting in blood specimen test tubes and kidney dialysis machines.

Ludwig Karl Martin Leonhard Albrecht Kossel was a German biochemist and pioneer in the study of genetics. He was awarded the Nobel Prize for Physiology or Medicine in 1910 for his work in determining the chemical composition of nucleic acids, the genetic substance of biological cells.

Cardiopulmonary Bypass (CPB) is a machine that temporarily takes over the function of the heart and lungs during a cardiac surgery by maintaining the circulation of blood and oxygen throughout the body.

Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion.

In biochemistry, a ribonucleotide is a nucleotide containing ribose as its pentose component. It is considered a molecular precursor of nucleic acids. Nucleotides are the basic building blocks of DNA and RNA. Ribonucleotides themselves are basic monomeric building blocks for RNA. Deoxyribonucleotides, formed by reducing ribonucleotides with the enzyme ribonucleotide reductase (RNR), are essential building blocks for DNA. There are several differences between DNA deoxyribonucleotides and RNA ribonucleotides. Successive nucleotides are linked together via phosphodiester bonds.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and treatment of venous thromboembolism and in the treatment of myocardial infarction.

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.
Ximelagatran is an anticoagulant that has been investigated extensively as a replacement for warfarin that would overcome the problematic dietary, drug interaction, and monitoring issues associated with warfarin therapy. In 2006, its manufacturer AstraZeneca announced that it would withdraw pending applications for marketing approval after reports of hepatotoxicity during trials, and discontinue its distribution in countries where the drug had been approved.
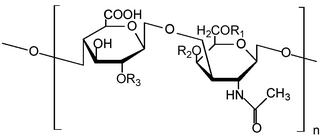
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
Protamines are small, arginine-rich, nuclear proteins that replace histones late in the haploid phase of spermatogenesis and are believed essential for sperm head condensation and DNA stabilization. They may allow for denser packaging of DNA in the spermatozoon than histones, but they must be decompressed before the genetic data can be used for protein synthesis. However, part of the sperm's genome is packaged by histones thought to bind genes that are essential for early embryonic development.

Enoxaparin sodium, sold under the brand name Lovenox among others, is an anticoagulant medication. It is used to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) including during pregnancy and following certain types of surgery. It is also used in those with acute coronary syndrome (ACS) and heart attacks. It is given by injection just under the skin or into a vein. It is also used during hemodialysis.

Hexadimethrine bromide is a cationic polymer with several uses. Currently, it is primarily used to increase the efficiency of transduction of certain cells with retrovirus in cell culture. Hexadimethrine bromide acts by neutralizing the charge repulsion between virions and sialic acid on the cell surface. Use of Polybrene can improve transduction efficiency 100-1000 fold although it can be toxic to some cell types. Polybrene in combination with DMSO shock is used to transfect some cell types such as NIH-3T3 and CHO. It has other uses, including a role in protein sequencing.

Cationic liposomes are spherical structures that contain positively charged lipids. Cationic liposomes can vary in size between 40 nm and 500 nm, and they can either have one lipid bilayer (monolamellar) or multiple lipid bilayers (multilamellar). The positive charge of the phospholipids allows cationic liposomes to form complexes with negatively charged nucleic acids through ionic interactions. Upon interacting with nucleic acids, cationic liposomes form clusters of aggregated vesicles. These interactions allow cationic liposomes to condense and encapsulate various therapeutic and diagnostic agents in their aqueous compartment or in their lipid bilayer. These cationic liposome-nucleic acid complexes are also referred to as lipoplexes. Due to the overall positive charge of cationic liposomes, they interact with negatively charged cell membranes more readily than classic liposomes. This positive charge can also create some issues in vivo, such as binding to plasma proteins in the bloodstream, which leads to opsonization. These issues can be reduced by optimizing the physical and chemical properties of cationic liposomes through their lipid composition. Cationic liposomes are increasingly being researched for use as delivery vectors in gene therapy due to their capability to efficiently transfect cells. A common application for cationic liposomes is cancer drug delivery.
Iduronidase, sold as Aldurazyme, is an enzyme with the systematic name glycosaminoglycan α-L-iduronohydrolase. It catalyses the hydrolysis of unsulfated α-L-iduronosidic linkages in dermatan sulfate.
Eosinophil cationic protein (ECP) also known as ribonuclease 3 is a basic protein located in the eosinophil primary matrix. In humans, the eosinophil cationic protein is encoded by the RNASE3 gene.

Gene therapy utilizes the delivery of DNA into cells, which can be accomplished by several methods, summarized below. The two major classes of methods are those that use recombinant viruses and those that use naked DNA or DNA complexes.

A majority of the human genome is made up of non-protein coding DNA. It infers that such sequences are not commonly employed to encode for a protein. However, even though these regions do not code for protein, they have other functions and carry necessary regulatory information.They can be classified based on the size of the ncRNA. Small noncoding RNA is usually categorized as being under 200 bp in length, whereas long noncoding RNA is greater than 200bp. In addition, they can be categorized by their function within the cell; Infrastructural and Regulatory ncRNAs. Infrastructural ncRNAs seem to have a housekeeping role in translation and splicing and include species such as rRNA, tRNA, snRNA.Regulatory ncRNAs are involved in the modification of other RNAs.
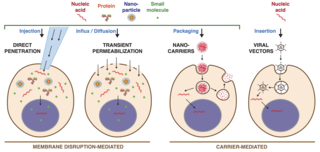
Intracellular delivery is the process of introducing external materials into living cells. Materials that are delivered into cells include nucleic acids, proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects. Such molecules and materials can be used to investigate cellular behavior, engineer cell operations or correct a pathological function.
















