Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations Na+ and hydroxide anions OH−.

Brine is water with a high-concentration solution of salt. In diverse contexts, brine may refer to the salt solutions ranging from about 3.5% up to about 26%. Brine forms naturally due to evaporation of ground saline water but it is also generated in the mining of sodium chloride. Brine is used for food processing and cooking, for de-icing of roads and other structures, and in a number of technological processes. It is also a by-product of many industrial processes, such as desalination, so it requires wastewater treatment for proper disposal or further utilization.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.

Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption, but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.

Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.

Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as the relative quantities of the other constituents are known.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate:

Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins, but is increasingly being accomplished using nanofiltration or reverse osmosis membranes.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.

Ammonium chlorate is an inorganic compound with the formula NH4ClO3.

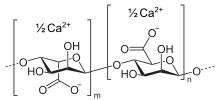
Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. With metals such as sodium and calcium, its salts are known as alginates. Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular, or powdered forms.

Easy Cheese is the trademark for a processed cheese spread product distributed by Mondelēz International. It is also commonly referred to by generic terms such as "spray cheese", "squirt cheese" or "cheese in a can". Easy Cheese is packaged in a metal can filled with air covered with a plastic cap that reveals a straight, flexible nozzle where the cheese is extruded.

A thickening agent or thickener is a substance which can increase the viscosity of a liquid without substantially changing its other properties. Edible thickeners are commonly used to thicken sauces, soups, and puddings without altering their taste; thickeners are also used in paints, inks, explosives, and cosmetics.

In chemical engineering, biochemical engineering and protein purification, crossflow filtration is a type of filtration. Crossflow filtration is different from dead-end filtration in which the feed is passed through a membrane or bed, the solids being trapped in the filter and the filtrate being released at the other end. Cross-flow filtration gets its name because the majority of the feed flow travels tangentially across the surface of the filter, rather than into the filter. The principal advantage of this is that the filter cake is substantially washed away during the filtration process, increasing the length of time that a filter unit can be operational. It can be a continuous process, unlike batch-wise dead-end filtration.
A vacuum ceramic filter is designed to separate liquids from solids for dewatering of ore concentrates purposes. The device consists of a rotator, slurry tank, ceramic filter plate, distributor, discharge scraper, cleaning device, frame, agitating device, pipe system, vacuum system, automatic acid dosing system, automatic lubricating system, valve and discharge chute. The operation and construction principle of vacuum ceramic filter resemble those of a conventional disc filter, but the filter medium is replaced by a finely porous ceramic disc. The disc material is inert, has a long operational life and is resistant to almost all chemicals. Performance can be optimized by taking into account all those factors which affect the overall efficiency of the separation process. Some of the variables affecting the performance of a vacuum ceramic filter include the solid concentration, speed rotation of the disc, slurry level in the feed basin, temperature of the feed slurry, and the pressure during dewatering stages and filter cake formation.
An alginate dressing is a natural wound dressing derived from carbohydrate sources released by clinical bacterial species, in the same manner as biofilm formation. These types of dressings are best used on wounds that have a large amount of exudate. They may be used on full-thickness burns, surgical wounds, split-thickness graft donor sites, Mohs surgery defects, refractory decubiti, and chronic ulcers. They can also be applied onto dry wounds after normal saline is first applied to the site of application.
Clarifying agents are used to remove suspended solids from liquids by inducing flocculation, causing the solids to form larger aggregates that can be easily removed after they either float to the surface or sink to the bottom of the containment vessel.

A water-gel explosive is a fuel sensitized explosive mixture consisting of an aqueous ammonium nitrate solution that acts as the oxidizer. Water gels that are cap-insensitive are referred to under United States safety regulations as blasting agents. Water gel explosives have a jelly-like consistency and come in sausage-like packing stapled shut on both sides.
Radium compounds are compounds containing the element radium (Ra). Due to radium's radioactivity, not many compounds have been well characterized. Solid radium compounds are white as radium ions provide no specific coloring, but they gradually turn yellow and then dark over time due to self-radiolysis from radium's alpha decay. Insoluble radium compounds coprecipitate with all barium, most strontium, and most lead compounds.