Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The process of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Disseminated intravascular coagulation (DIC) is a condition in which blood clots form throughout the body, blocking small blood vessels. Symptoms may include chest pain, shortness of breath, leg pain, problems speaking, or problems moving parts of the body. As clotting factors and platelets are used up, bleeding may occur. This may include blood in the urine, blood in the stool, or bleeding into the skin. Complications may include organ failure.

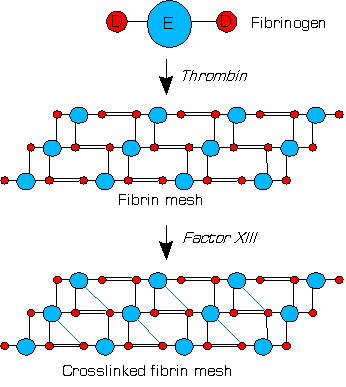
Fibrin is a fibrous, non-globular protein involved in the clotting of blood. It is formed by the action of the protease thrombin on fibrinogen, which causes it to polymerize. The polymerized fibrin, together with platelets, forms a hemostatic plug or clot over a wound site.

Fibrinogen is a glycoprotein complex, produced in the liver, that circulates in the blood of all vertebrates. During tissue and vascular injury, it is converted enzymatically by thrombin to fibrin and then to a fibrin-based blood clot. Fibrin clots function primarily to occlude blood vessels to stop bleeding. Fibrin also binds and reduces the activity of thrombin. This activity, sometimes referred to as antithrombin I, limits clotting. Fibrin also mediates blood platelet and endothelial cell spreading, tissue fibroblast proliferation, capillary tube formation, and angiogenesis and thereby promotes revascularization and wound healing.

Prothrombin is encoded in the human by the F2 gene. It is proteolytically cleaved during the clotting process by the prothrombinase enzyme complex to form thrombin.
Fibrinolysis is a process that prevents blood clots from growing and becoming problematic. Primary fibrinolysis is a normal body process, while secondary fibrinolysis is the breakdown of clots due to a medicine, a medical disorder, or some other cause.

Coagulation factor XII, also known as Hageman factor, is a plasma protein involved in coagulation. It is the zymogen form of factor XIIa, an enzyme of the serine protease class. In humans, factor XII is encoded by F12 gene.
D-dimer is a dimer that is a fibrin degradation product, a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two D fragments of the fibrin protein joined by a cross-link, hence forming a protein dimer.

Coagulation factor X, or Stuart factor, is an enzyme of the coagulation cascade, encoded in humans by F10 gene. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
Coagulation factor V, also less commonly known as proaccelerin or labile factor, is a protein involved in coagulation, encoded, in humans, by F5 gene. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Factor V deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.

Factor XI, or plasma thromboplastin antecedent, is the zymogen form of factor XIa, one of the enzymes involved in coagulation. Like many other coagulation factors, it is a serine protease. In humans, factor XI is encoded by F11 gene.

Hirudin is a naturally occurring peptide in the salivary glands of blood-sucking leeches that has a blood anticoagulant property. This is essential for the leeches' habit of feeding on blood, since it keeps a host's blood flowing after the worm's initial puncture of the skin.
Factor XIII deficiency occurs exceedingly rarely, causing a severe bleeding tendency. The incidence is one in a million to one in five million people, with higher incidence in areas with consanguineous marriage such as Iran that has the highest global incidence of the disorder. Most are due to mutations in the A subunit gene. This mutation is inherited in an autosomal recessive fashion.

Coagulation factor XIII A chain, (FXIIIa) is a protein that in humans is encoded by the F13A1 gene.

Coagulation factor XIII B chain is a protein that in humans is encoded by the F13B gene.
Clotting time is a general term for the time required for a sample of blood to form a clot, or, in medical terms, coagulate. The term "clotting time" is often used when referring to tests such as the prothrombin time (PT), activated partial thromboplastin time, activated clotting time (ACT), thrombin time (TT), or Reptilase time. These tests are coagulation studies performed to assess the natural clotting ability of a sample of blood. In a clinical setting, healthcare providers will order one of these tests to evaluate a patient's blood for any abnormalities in the time it takes for their blood to clot. Each test involves adding a specific substance to the blood and measuring the time until the blood forms fibrin which is one of the first signs of clotted blood. Each test points to a different component of the clotting sequence which is made up of coagulation factors that help form clots. Abnormal results could be due to a number of reasons including, but, not limited to, deficiency in clotting factors, dysfunction of clotting factors, blood-thinning medications, medication side-effects, platelet deficiency, inherited bleeding or clotting disorders, liver disease, or advanced illness resulting in a medical emergency known as disseminated intravascular coagulation (DIC).

Fibrin glue is a surgical formulation used to create a fibrin clot for hemostasis, cartilage repair surgeries or wound healing. It contains separately packaged human fibrinogen and human thrombin.

Coagulin is a gel-forming protein of hemolymph that hinders the spread of bacterial and fungal invaders by immobilizing them. It is produced in the coagulogen form before being cleaved into the active form through a serine proteinase cascade. It has been most extensively studied in horseshoe crabs. It has also been produced by other organisms, such as Bacillus coagulans I4 in a plasmid location. In human medicine, coagulation of coagulin is the basis of detection of bacterial endotoxin through the Limulus amebocyte lysate test for parenteral medications.
Blood clotting tests are the tests used for diagnostics of the hemostasis system. Coagulometer is the medical laboratory analyzer used for testing of the hemostasis system. Modern coagulometers realize different methods of activation and observation of development of blood clots in blood or in blood plasma.
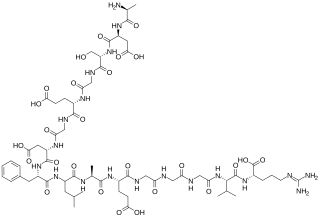
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen and are cleaved by the enzyme thrombin to convert fibrinogen into covalently-linked fibrin monomers. The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII, and these fibrin polymers form the backbone of a thrombus. Hence, the fibrinopeptides are sensitive markers of fibrinogenesis, thrombin activity, and coagulation.