
Chromium is a chemical element; it has symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.

Selenium is a chemical element; it has symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excreted by cells to create non-cellular structures, such as hair, scales, feathers, or exoskeletons. Some nutrients can be metabolically converted to smaller molecules in the process of releasing energy, such as for carbohydrates, lipids, proteins, and fermentation products, leading to end-products of water and carbon dioxide. All organisms require water. Essential nutrients for animals are the energy sources, some of the amino acids that are combined to create proteins, a subset of fatty acids, vitamins and certain minerals. Plants require more diverse minerals absorbed through roots, plus carbon dioxide and oxygen absorbed through leaves. Fungi live on dead or living organic matter and meet nutrient needs from their host.

In the context of nutrition, a mineral is a chemical element. Some "minerals" are essential for life, most are not. Minerals are one of the four groups of essential nutrients, the others of which are vitamins, essential fatty acids, and essential amino acids. The five major minerals in the human body are calcium, phosphorus, potassium, sodium, and magnesium. The remaining elements are called "trace elements". The generally accepted trace elements are iron, chlorine, cobalt, copper, zinc, manganese, molybdenum, iodine, and selenium; there is some evidence that there may be more.

Selenomethionine (SeMet) is a naturally occurring amino acid. The L-selenomethionine enantiomer is the main form of selenium found in Brazil nuts, cereal grains, soybeans, and grassland legumes, while Se-methylselenocysteine, or its γ-glutamyl derivative, is the major form of selenium found in Astragalus, Allium, and Brassica species. In vivo, selenomethionine is randomly incorporated instead of methionine. Selenomethionine is readily oxidized.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.

Selenite refers to the anion with the chemical formula SeO2−3. It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, SO2−3. Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite Na2SeO3 which is a common source of selenite. Selenite also refers to the esters of selenous acid, for example dimethyl selenite (CH3)2SeO3.

Sodium tellurite is an inorganic tellurium compound with formula Na2TeO3. It is a water-soluble white solid and a weak reducing agent. Sodium tellurite is an intermediate in the extraction of the element, tellurium; it is a product obtained from anode slimes and is a precursor to tellurium.

Vanadyl(IV) sulfate describes a collection of inorganic compounds of vanadium with the formula, VOSO4(H2O)x where 0 ≤ x ≤ 6. The pentahydrate is common. This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability. It features the vanadyl ion, VO2+, which has been called the "most stable diatomic ion".

Selenium compounds are compounds containing the element selenium (Se). Among these compounds, selenium has various oxidation states, the most common ones being −2, +4, and +6. Selenium compounds exist in nature in the form of various minerals, such as clausthalite, guanajuatite, tiemannite, crookesite etc., and can also coexist with sulfide minerals such as pyrite and chalcopyrite. For many mammals, selenium compounds are essential. For example, selenomethionine and selenocysteine are selenium-containing amino acids present in the human body. Selenomethionine participates in the synthesis of selenoproteins. The reduction potential and pKa (5.47) of selenocysteine are lower than those of cysteine, making some proteins have antioxidant activity. Selenium compounds have important applications in semiconductors, glass and ceramic industries, medicine, metallurgy and other fields.
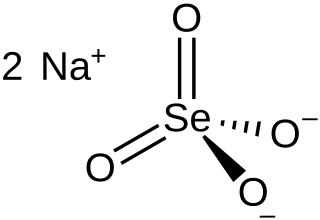
Sodium selenate is the inorganic compound with the formula Na
2SeO
4, not to be confused with sodium selenite. It exists as the anhydrous salt, the heptahydrate, and the decahydrate. These are white, water-soluble solids. The decahydrate is a common ingredient in multivitamins and livestock feed as a source of selenium. The anhydrous salt is used in the production of some glass. Although the selenates are much more toxic, many physical properties of sodium selenate and sodium sulfate are similar.
Selenium deficiency occurs when an organism lacks the required levels of selenium, a critical nutrient in many species. Deficiency, although relatively rare in healthy well-nourished individuals, can have significant negative results, affecting the health of the heart and the nervous system; contributing to depression, anxiety, and dementia; and interfering with reproduction and gestation.
A co-carcinogen is a chemical that promotes the effects of a carcinogen in the production of cancer. Usually, the term is used to refer to chemicals that are not carcinogenic on their own, such that an equivalent amount of the chemical is insufficient to initiate carcinogenesis. A chemical can be co-carcinogenic with other chemicals or with nonchemical carcinogens, such as UV radiation.
Iodine is an essential trace element in biological systems. It has the distinction of being the heaviest element commonly needed by living organisms as well as the second-heaviest known to be used by any form of life. It is a component of biochemical pathways in organisms from all biological kingdoms, suggesting its fundamental significance throughout the evolutionary history of life.
Selenium yeast is a feed additive for livestock, used to increase the selenium content in their fodder. It is a form of selenium currently approved for human consumption in the EU and Britain. Inorganic forms of selenium are used in feeds. Since these products can be patented, producers can demand premium prices. It is produced by fermenting Saccharomyces cerevisiae in a selenium-rich media.

Selenium is an essential micronutrient for animals, though it is toxic in large doses. In plants, it sometimes occurs in toxic amounts as forage, e.g. locoweed. Selenium is a component of the amino acids selenocysteine and selenomethionine. In humans, selenium is a trace element nutrient that functions as cofactor for glutathione peroxidases and certain forms of thioredoxin reductase. Selenium-containing proteins are produced from inorganic selenium via the intermediacy of selenophosphate (PSeO33−).

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.
A selenate selenite is a chemical compound or salt that contains selenite and selenate anions (SeO32- and SeO42-). These are mixed anion compounds. Some have third anions.
















