
Ascorbic acid is an organic compound with formula C
6H
8O
6, originally called hexuronic acid. It is a white solid, but impure samples can appear yellowish. It dissolves freely in water to give mildly acidic solutions. It is a mild reducing agent.

Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit visual deterioration, such as the enzymatic browning reaction in apples after they are cut during food preparation. By preserving food, food waste can be reduced, which is an important way to decrease production costs and increase the efficiency of food systems, improve food security and nutrition and contribute towards environmental sustainability. For instance, it can reduce the environmental impact of food production.
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or by undesirable chemical changes. In general, preservation is implemented in two modes, chemical and physical. Chemical preservation entails adding chemical compounds to the product. Physical preservation entails processes such as refrigeration or drying. Preservative food additives reduce the risk of foodborne infections, decrease microbial spoilage, and preserve fresh attributes and nutritional quality. Some physical techniques for food preservation include dehydration, UV-C radiation, freeze-drying, and refrigeration. Chemical preservation and physical preservation techniques are sometimes combined.
Rancidification is the process of complete or incomplete autoxidation or hydrolysis of fats and oils when exposed to air, light, moisture, or bacterial action, producing short-chain aldehydes, ketones and free fatty acids.

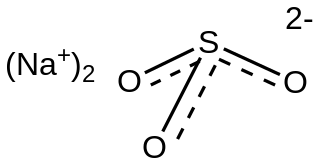
Sulfites or sulphites are compounds that contain the sulfite ion, SO2−
3. The sulfite ion is the conjugate base of bisulfite. Although its acid is elusive, its salts are widely used.

Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as an antioxidant and preservative. It is also suitable for the softening of lignin in the pulping and refining processes of wood and lignocellulosic materials. A heptahydrate is also known but it is less useful because of its greater susceptibility toward oxidation by air.

Erythorbic acid is a stereoisomer of ascorbic acid. It is synthesized by a reaction between methyl 2-keto-D-gluconate and sodium methoxide. It can also be synthesized from sucrose or by strains of Penicillium that have been selected for this feature. It is denoted by E number E315, and is widely used as an antioxidant in processed foods.
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure.

Potassium sorbate is the potassium salt of sorbic acid, chemical formula CH3CH=CH−CH=CH−CO2K. It is a white salt that is very soluble in water (58.2% at 20 °C). It is primarily used as a food preservative (E number 202). Potassium sorbate is effective in a variety of applications including food, wine, and personal-care products. While sorbic acid occurs naturally in rowan and hippophae berries, virtually all of the world's supply of sorbic acid, from which potassium sorbate is derived, is manufactured synthetically.

Mineral ascorbates are a group of salts of ascorbic acid. They are composed of a mineral cation bonded to ascorbate.

Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization.

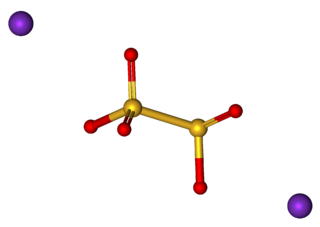
A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O2−
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite HSO−
3 anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite.
Warmed-over flavor is an unpleasant characteristic usually associated with meat which has been cooked and then refrigerated. The deterioration of meat flavor is most noticeable upon reheating. As cooking and subsequent refrigeration is the case with most convenience foods containing meat, it is a significant challenge to the processed food industry. The flavor is variously described as "rancid," "stale," and like "cardboard," and even compared to "damp dog hair." Warmed-over flavor is caused by the oxidative decomposition of lipids in the meat into chemicals which have an unpleasant taste or odor. This decomposition process begins after cooking or processing and is aided by the release of naturally occurring iron in the meat.

Potassium erythorbate (C6H7KO6) is a food additive. Chemically, it is the potassium salt of erythorbic acid. As an antioxidant structurally related to vitamin C, it helps improve flavor stability and prevents the formation of carcinogenic nitrosamines.

Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3.

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such as a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry.

Wine preservatives are used to preserve the quality and shelf life of bottled wine without affecting its taste. Specifically, they are used to prevent oxidation and bacterial spoilage by inhibiting microbial activity.
The topic of sulfite food and beverage additives covers the application of sulfites in food chemistry. "Sulfite" is jargon that encompasses a variety of materials that are commonly used as preservatives or food additive in the production of diverse foods and beverages. Although sulfite salts are relatively nontoxic, their use has led to controversy, resulting in extensive regulations. Sulfites are a source of sulfur dioxide (SO2), a bactericide.















