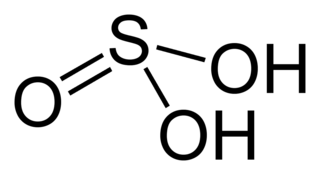
Sulfuric(IV) acid, also known as sulfurous (UK: sulphurous) acid and thionic acid, is the chemical compound with the formula H2SO3.

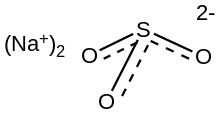
Sulfites or sulphites are compounds that contain the sulfite ion, SO2−
3. The sulfite ion is the conjugate base of bisulfite. Although its acid is elusive, its salts are widely used.

Sodium metabisulfite or sodium pyrosulfite (IUPAC spelling; Br. E. sodium metabisulphite or sodium pyrosulphite) is an inorganic compound of chemical formula Na2S2O5. The substance is sometimes referred to as disodium metabisulfite. It is used as a disinfectant, antioxidant, and preservative agent. When dissolved in water it forms sodium bisulfite.

Sodium thiosulfate is an inorganic compound with the formula Na2S2O3·(H2O)(x) .Typically it is available as the white or colorless pentahydrate, It is a white solid that dissolves well in water. The compound is a reducing agent and a ligand, and these properties underpin its applications.

Flue-gas desulfurization (FGD) is a set of technologies used to remove sulfur dioxide from exhaust flue gases of fossil-fuel power plants, and from the emissions of other sulfur oxide emitting processes such as waste incineration, petroleum refineries, cement and lime kilns.
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called comproportionation, also known as synproportionation.

Sodium dithionite is a white crystalline powder with a sulfurous odor. Although it is stable in dry air, it decomposes in hot water and in acid solutions.
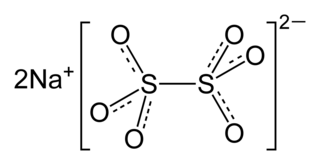
Sodium dithionate Na2S2O6 is an important compound for inorganic chemistry. It is also known under names disodium dithionate, sodium hyposulfate, and sodium metabisulfate. The sulfur can be considered to be in its +5 oxidation state.

The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO−
3. Salts containing the HSO−
3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+HSO−
3.
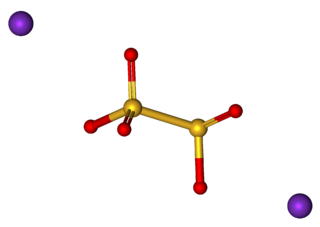
Potassium metabisulfite, K2S2O5, also known as potassium pyrosulfite, is a white crystalline powder with a pungent odour. It is mainly used as an antioxidant or chemical sterilant. As a disulfite, it is chemically very similar to sodium metabisulfite, with which it is sometimes used interchangeably. Potassium metabisulfite has a monoclinic crystal structure.

The dithionite is the oxyanion with the formula [S2O4]2−. It is commonly encountered as the salt sodium dithionite. For historical reasons, it is sometimes called hydrosulfite, but it contains no hydrogen and is not a sulfite. The dianion has a steric number of 4 and trigonal pyramidal geometry.

Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximate chemical formula KHSO3. Potassium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of potassium ions and bisulfite ions. It is a white solid with an odor of sulfur dioxide. Attempts to crystallize potassium bisulfite yield potassium metabisulfite, K2S2O5.
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These chemicals cleave the bonds between the cellulose and lignin components of the lignocellulose. A variety of sulfite/bisulfite salts are used, including sodium (Na+), calcium (Ca2+), potassium (K+), magnesium (Mg2+), and ammonium (NH4+). The lignin is converted to lignosulfonates, which are soluble and can be separated from the cellulose fibers. For the production of cellulose, the sulfite process competes with the Kraft process which produces stronger fibers and is less environmentally costly.

Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization.
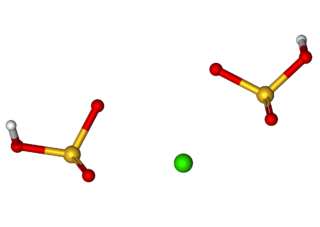
Calcium bisulfite is an inorganic compound which is the salt of a calcium cation and a bisulfite anion. It may be prepared by treating lime with an excess of sulfur dioxide and water. As a food additive it is used as a preservative under the E number E227. Calcium bisulfite is an acid salt and behaves like an acid in aqueous solution. It is used in the sulfite process for producing paper from wood chips.
The Wellman–Lord process is a regenerable process to remove sulfur dioxide from flue gas without creating a throwaway sludge product.

A disulfite, commonly known as metabisulfite or pyrosulfite, is a chemical compound containing the ion S
2O2−
5. It is a colorless dianion that is primarily marketed in the form of sodium metabisulfite or potassium metabisulfite. When dissolved in water, these salts release the hydrogensulfite HSO−
3 anion. These salts act equivalently to sodium hydrogensulfite or potassium hydrogensulfite.

Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3.

Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite in fact is not a real compound, but a mixture of salts that dissolve in water to give solutions composed of sodium and bisulfite ions. It appears in form of white or yellowish-white crystals with an odor of sulfur dioxide. Regardless of its ill-defined nature, sodium bisulfite is used in many different industries such as a food additive with E number E222 in the food industry, a reducing agent in the cosmetic industry, and a decomposer of residual hypochlorite used in the bleaching industry.
The topic of sulfite food and beverage additives covers the application of sulfites in food chemistry. "Sulfite" is jargon that encompasses a variety of materials that are commonly used as preservatives or food additive in the production of diverse foods and beverages. Although sulfite salts are relatively nontoxic, their use has led to controversy, resulting in extensive regulations. Sulfites are a source of sulfur dioxide (SO2), a bactericide.