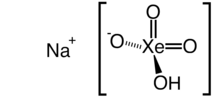The noble gases make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.

Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.
In chemistry, perxenates are salts of the yellow xenon-containing anion XeO4−
6. This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°. The Xe–O bond length was determined by X-ray crystallography to be 1.875 Å.
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A half-neutralization occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions to create water molecules.

Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
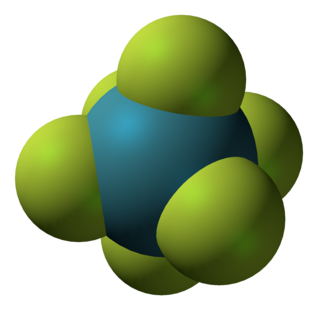
Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon that have been studied experimentally, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.

Sodium oxalate, or disodium oxalate, is the sodium salt of oxalic acid with the formula Na2C2O4. It is a white, crystalline, odorless solid, that decomposes above 290 °C.

Xenon trioxide is an unstable compound of xenon in its +6 oxidation state. It is a very powerful oxidizing agent, and liberates oxygen from water slowly, accelerated by exposure to sunlight. It is dangerously explosive upon contact with organic materials. When it detonates, it releases xenon and oxygen gas.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.
In chemistry, hypomanganate, also called manganate(V) or tetraoxidomanganate(3−), is a trivalent anion (negative ion) composed of manganese and oxygen, with formula MnO3−
4.
Xenic acid is a proposed noble gas compound with the chemical formula H2XeO4 or XeO2(OH)2. It has not been isolated, and the published characterization data are ambiguous.

Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.

Barium ferrate is the chemical compound of formula BaFeO4. This is a rare compound containing iron in the +6 oxidation state. The ferrate(VI) ion has two unpaired electrons, making it paramagnetic. It is isostructural with BaSO4, and contains the tetrahedral [FeO4]2− anion.

Manganese(II) molybdate is an inorganic compound with the chemical formula MnMoO4. α-MnMoO4 has a monoclinic crystal structure. It is also antiferromagnetic at low temperatures.

Pentafluorosulfanylbenzene, or phenylsulfur pentafluoride, is an organosulfur compound with the formula C6H5SF5. It is colorless liquid with high chemical stability.

Radon compounds are compounds formed by the element radon (Rn). Radon is a member of the zero-valence elements that are called noble gases, and is chemically not very reactive. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas at standard conditions, unlike its decay-chain parents, it can readily be extracted from them for research.