
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin calx "lime", which was obtained from heating limestone.

Limestone is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of CaCO3. Limestone forms when these minerals precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life.

Kidney stone disease, also known as nephrolithiasis or urolithiasis, is a crystallopathy where a solid piece of material develops in the urinary tract. Kidney stones typically form in the kidney and leave the body in the urine stream. A small stone may pass without causing symptoms. If a stone grows to more than 5 millimeters, it can cause blockage of the ureter, resulting in sharp and severe pain in the lower back or abdomen. A stone may also result in blood in the urine, vomiting, or painful urination. About half of people who have had a kidney stone are likely to have another within ten years.

Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula CaC2O4. It forms hydrates CaC2O4·nH2O, where n varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydrate CaC2O4·H2O occurs naturally as the mineral whewellite, forming envelope-shaped crystals, known in plants as raphides. The two rarer hydrates are dihydrate CaC2O4·2H2O, which occurs naturally as the mineral weddellite, and trihydrate CaC2O4·3H2O, which occurs naturally as the mineral caoxite, are also recognized. Some foods have high quantities of calcium oxalates and can produce sores and numbing on ingestion and may even be fatal. Cultural groups with diets that depend highly on fruits and vegetables high in calcium oxalate, such as those in Micronesia, reduce the level of it by boiling and cooking them. They are a constituent in 76% of human kidney stones. Calcium oxalate is also found in beerstone, a scale that forms on containers used in breweries.

Calcium carbide, also known as calcium acetylide, is a chemical compound with the chemical formula of CaC2. Its main use industrially is in the production of acetylene and calcium cyanamide.
Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as medications to decrease blood pressure in patients with hypertension. CCBs are particularly effective against large vessel stiffness, one of the common causes of elevated systolic blood pressure in elderly patients. Calcium channel blockers are also frequently used to alter heart rate, to prevent peripheral and cerebral vasospasm, and to reduce chest pain caused by angina pectoris.

Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation.

Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca(OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide.
Verapamil, sold under various trade names, is a calcium channel blocker medication used for the treatment of high blood pressure, angina, and supraventricular tachycardia. It may also be used for the prevention of migraines and cluster headaches. It is given by mouth or by injection into a vein.
Amlodipine, sold under the brand name Norvasc among others, is a calcium channel blocker medication used to treat high blood pressure and coronary artery disease. It is taken by mouth.

Calcium citrate is the calcium salt of citric acid. It is commonly used as a food additive (E333), usually as a preservative, but sometimes for flavor. In this sense, it is similar to sodium citrate. Calcium citrate is also found in some dietary calcium supplements. Calcium makes up 24.1% of calcium citrate (anhydrous) and 21.1% of calcium citrate (tetrahydrate) by mass. The tetrahydrate occurs in nature as the mineral Earlandite.

Diltiazem, sold under the brand name Cardizem among others, is a calcium channel blocker medication used to treat high blood pressure, angina, and certain heart arrhythmias. It may also be used in hyperthyroidism if beta blockers cannot be used. It is taken by mouth or injection into a vein. When given by injection, effects typically begin within a few minutes and last a few hours.
ATC code A12Mineral supplements is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products. Subgroup A12 is part of the anatomical group A Alimentary tract and metabolism.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. It is only recommended in those with significant abnormal heart rhythms due to potentially serious side effects. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or injection into a vein.

Hydroxyapatite, also called hydroxylapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but it is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. Hydroxyapatite is the hydroxyl endmember of the complex apatite group. The OH− ion can be replaced by fluoride, chloride or carbonate, producing fluorapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Pure hydroxyapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis.
Calcitriol is the active form of vitamin D, normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It is a hormone which binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes. Calcitriol increases blood calcium (Ca2+) mainly by increasing the uptake of calcium from the intestines.

Calcification is the accumulation of calcium salts in a body tissue. It normally occurs in the formation of bone, but calcium can be deposited abnormally in soft tissue, causing it to harden. Calcifications may be classified on whether there is mineral balance or not, and the location of the calcification. Calcification may also refer to the processes of normal mineral deposition in biological systems, such as the formation of stromatolites or mollusc shells.

The plasma membrane Ca2+ ATPase (PMCA) is a transport protein in the plasma membrane of cells and functions to remove calcium (Ca2+) from the cell. PMCA function is vital for regulating the amount of Ca2+ within all eukaryotic cells. There is a very large transmembrane electrochemical gradient of Ca2+ driving the entry of the ion into cells, yet it is very important that they maintain low concentrations of Ca2+ for proper cell signalling. Thus, it is necessary for cells to employ ion pumps to remove the Ca2+. The PMCA and the sodium calcium exchanger (NCX) are together the main regulators of intracellular Ca2+ concentrations. Since it transports Ca2+ into the extracellular space, the PMCA is also an important regulator of the calcium concentration in the extracellular space.
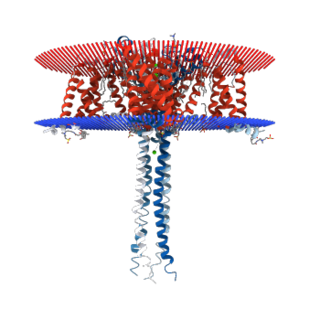
N-type calcium channels also called Cav2.2 channels are voltage gated calcium channels that are localized primarily on the nerve terminals and dendrites as well as neuroendocrine cells. The calcium N-channel consists of several subunits: the primary subunit α1B and the auxiliary subunits α2δ and β. The α1B subunit forms the pore through which the calcium enters and helps to determine most of the channel's properties. These channels play an important role in the neurotransmission during development. In the adult nervous system, N-type calcium channels are critically involved in the release of neurotransmitters, and in pain pathways. N-type calcium channels are the target of ziconotide, the drug prescribed to relieve intractable cancer pain. There are many known N-type calcium channel blockers that function to inhibit channel activity, although the most notable blockers are ω-conotoxins.


















