Kidney stone disease, also known as renal calculus disease, nephrolithiasis or urolithiasis, is a crystallopathy where a solid piece of material develops in the urinary tract. Renal calculi typically form in the kidney and leave the body in the urine stream. A small calculus may pass without causing symptoms. If a stone grows to more than 5 millimeters, it can cause blockage of the ureter, resulting in sharp and severe pain in the lower back or abdomen. A calculus may also result in blood in the urine, vomiting, or painful urination. About half of people who have had a renal calculus are likely to have another within ten years.

Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
The excretory system is a passive biological system that removes excess, unnecessary materials from the body fluids of an organism, so as to help maintain internal chemical homeostasis and prevent damage to the body. The dual function of excretory systems is the elimination of the waste products of metabolism and to drain the body of used up and broken down components in a liquid and gaseous state. In humans and other amniotes, most of these substances leave the body as urine and to some degree exhalation, mammals also expel them through sweating.

Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula CaC2O4 or Ca(COO)2. It forms hydrates CaC2O4·nH2O, where n varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydrate CaC2O4·H2O occurs naturally as the mineral whewellite, forming envelope-shaped crystals, known in plants as raphides. The two rarer hydrates are dihydrate CaC2O4·2H2O, which occurs naturally as the mineral weddellite, and trihydrate CaC2O4·3H2O, which occurs naturally as the mineral caoxite, are also recognized. Some foods have high quantities of calcium oxalates and can produce sores and numbing on ingestion and may even be fatal. Cultural groups with diets that depend highly on fruits and vegetables high in calcium oxalate, such as those in Micronesia, reduce the level of it by boiling and cooking them. They are a constituent in 76% of human kidney stones. Calcium oxalate is also found in beerstone, a scale that forms on containers used in breweries.
Diuresis is the excretion of urine, especially when excessive (polyuria). The term collectively denotes the physiologic processes underpinning increased urine production by the kidneys during maintenance of fluid balance.

Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as "sodium citrate", though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor, and is a mild alkali.

Chlortalidone, also known as chlorthalidone, is a thiazide-like diuretic drug used to treat high blood pressure, swelling, diabetes insipidus, and renal tubular acidosis. Because chlortalidone is effective in most patients with high blood pressure, it is considered a preferred initial treatment. It is also used to prevent calcium-based kidney stones. It is taken by mouth. Effects generally begin within three hours and last for up to three days. Long-term treatment with chlortalidone is more effective than hydrochlorothiazide for prevention of heart attack or stroke.

Renal tubular acidosis (RTA) is a medical condition that involves an accumulation of acid in the body due to a failure of the kidneys to appropriately acidify the urine. In renal physiology, when blood is filtered by the kidney, the filtrate passes through the tubules of the nephron, allowing for exchange of salts, acid equivalents, and other solutes before it drains into the bladder as urine. The metabolic acidosis that results from RTA may be caused either by insufficient secretion of hydrogen ions into the latter portions of the nephron or by failure to reabsorb sufficient bicarbonate ions from the filtrate in the early portion of the nephron. Although a metabolic acidosis also occurs in those with chronic kidney disease, the term RTA is reserved for individuals with poor urinary acidification in otherwise well-functioning kidneys. Several different types of RTA exist, which all have different syndromes and different causes. RTA is usually an incidental finding based on routine blood draws that show abnormal results. Clinically, patients may present with vague symptoms such as dehydration, mental status changes, or delayed growth in adolescents.

Hemorrhagic cystitis or haemorrhagic cystitis is an inflammation of the bladder defined by lower urinary tract symptoms that include dysuria, hematuria, and hemorrhage. The disease can occur as a complication of cyclophosphamide, ifosfamide and radiation therapy. In addition to hemorrhagic cystitis, temporary hematuria can also be seen in bladder infection or in children as a result of viral infection.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Bladder stones or uroliths are a common occurrence in animals, especially in domestic animals such as dogs and cats. Occurrence in other species, including tortoises, has been reported as well. The stones form in the urinary bladder in varying size and numbers secondary to infection, dietary influences, and genetics. Stones can form in any part of the urinary tract in dogs and cats, but unlike in humans, stones of the kidney are less common and do not often cause significant disease, although they can contribute to pyelonephritis and chronic kidney disease. Types of stones include struvite, calcium oxalate, urate, cystine, calcium phosphate, and silicate. Struvite and calcium oxalate stones are by far the most common. Bladder stones are not the same as bladder crystals but if the crystals coalesce unchecked in the bladder they can become stones.

Travoprost, sold under the brand name Travatan among others, is a medication used to treat high pressure inside the eye including glaucoma. Specifically it is used for open angle glaucoma when other agents are not sufficient. It is used as an eye drop. Effects generally occur within two hours.

Monosodium citrate, more correctly, sodium dihydrogen citrate (Latin: natrium citricum acidulatum), is an acid salt of citric acid. Disodium citrate and trisodium citrate are also known. It can be prepared by partial neutralisation of citric acid with an aqueous solution of sodium bicarbonate or carbonate. It has a slightly acidic taste.

Magnesium citrate is a magnesium preparation in salt form with citric acid in a 1:1 ratio. It contains 11.23% magnesium by weight.

Nephrocalcinosis, once known as Albright's calcinosis after Fuller Albright, is a term originally used to describe the deposition of poorly soluble calcium salts in the renal parenchyma due to hyperparathyroidism. The term nephrocalcinosis is used to describe the deposition of both calcium oxalate and calcium phosphate. It may cause acute kidney injury. It is now more commonly used to describe diffuse, fine, renal parenchymal calcification in radiology. It is caused by multiple different conditions and is determined by progressive kidney dysfunction. These outlines eventually come together to form a dense mass. During its early stages, nephrocalcinosis is visible on x-ray, and appears as a fine granular mottling over the renal outlines. It is most commonly seen as an incidental finding with medullary sponge kidney on an abdominal x-ray. It may be severe enough to cause renal tubular acidosis or even end stage kidney disease, due to disruption of the kidney tissue by the deposited calcium salts.
Sodium citrate may refer to any of the sodium salts of citric acid :

Distal renal tubular acidosis (dRTA) is the classical form of RTA, being the first described. Distal RTA is characterized by a failure of acid secretion by the alpha intercalated cells of the distal tubule and cortical collecting duct of the distal nephron. This failure of acid secretion may be due to a number of causes. It leads to relatively alkaline urine, due to the kidney's inability to acidify the urine to a pH of less than 5.3.

Renal stone formation and passage during space flight can potentially pose a severe risk to crew member health and safety and could affect mission outcome. Although renal stones are routinely and successfully treated on Earth, the occurrence of these during space flight can prove to be problematic.

Calcium supplements are salts of calcium used in a number of conditions. Supplementation is generally only required when there is not enough calcium in the diet. By mouth they are used to treat and prevent low blood calcium, osteoporosis, and rickets. By injection into a vein they are used for low blood calcium that is resulting in muscle spasms and for high blood potassium or magnesium toxicity.
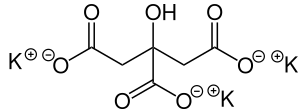
Alkali citrate is an inhibitor of kidney stones. It is used to increase urine citrate levels - this prevents calcium oxalate stones by binding to calcium and inhibiting its binding to oxalate. It is also used to increase urine pH - this prevents uric acid stones and cystine stones.