
Selenium is a chemical element; it has the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in Earth's crust. Selenium was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broad sense.

Wilson's disease is a genetic disorder in which excess copper builds up in the body. Symptoms are typically related to the brain and liver. Liver-related symptoms include vomiting, weakness, fluid build-up in the abdomen, swelling of the legs, yellowish skin, and itchiness. Brain-related symptoms include tremors, muscle stiffness, trouble in speaking, personality changes, anxiety, and psychosis.

Arsenic poisoning is a medical condition that occurs due to elevated levels of arsenic in the body. If arsenic poisoning occurs over a brief period of time, symptoms may include vomiting, abdominal pain, encephalopathy, and watery diarrhea that contains blood. Long-term exposure can result in thickening of the skin, darker skin, abdominal pain, diarrhea, heart disease, numbness, and cancer.

Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage.

Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type, dose, method, and duration of exposure. They may include muscle weakness, poor coordination, numbness in the hands and feet, skin rashes, anxiety, memory problems, trouble speaking, trouble hearing, or trouble seeing. High-level exposure to methylmercury is known as Minamata disease. Methylmercury exposure in children may result in acrodynia in which the skin becomes pink and peels. Long-term complications may include kidney problems and decreased intelligence. The effects of long-term low-dose exposure to methylmercury are unclear.
Gyromitrin is a toxin and carcinogen present in several members of the fungal genus Gyromitra, like G. esculenta. Its formula is C4H8N2O. It is unstable and is easily hydrolyzed to the toxic compound monomethylhydrazine CH3NHNH2. Monomethylhydrazine acts on the central nervous system and interferes with the normal use and function of vitamin B6. Poisoning results in nausea, stomach cramps, and diarrhea, while severe poisoning can result in convulsions, jaundice, or even coma or death. Exposure to monomethylhydrazine has been shown to be carcinogenic in small mammals.

Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. Ethion is known to affect a neural enzyme called acetylcholinesterase and prevent it from working.

Cyanotoxins are toxins produced by cyanobacteria. Cyanobacteria are found almost everywhere, but particularly in lakes and in the ocean where, under high concentration of phosphorus conditions, they reproduce exponentially to form blooms. Blooming cyanobacteria can produce cyanotoxins in such concentrations that they can poison and even kill animals and humans. Cyanotoxins can also accumulate in other animals such as fish and shellfish, and cause poisonings such as shellfish poisoning.
Manganism or manganese poisoning is a toxic condition resulting from chronic exposure to manganese. It was first identified in 1837 by James Couper.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.

Endrin is an organochlorine compound with the chemical formula C12H8Cl6O that was first produced in 1950 by Shell and Velsicol Chemical Corporation. It was primarily used as an insecticide, as well as a rodenticide and piscicide. It is a colourless, odorless solid, although commercial samples are often off-white. Endrin was manufactured as an emulsifiable solution known commercially as Endrex. The compound became infamous as a persistent organic pollutant and for this reason it is banned in many countries.

Soil contamination, soil pollution, or land pollution as a part of land degradation is caused by the presence of xenobiotic (human-made) chemicals or other alteration in the natural soil environment. It is typically caused by industrial activity, agricultural chemicals or improper disposal of waste. The most common chemicals involved are petroleum hydrocarbons, polynuclear aromatic hydrocarbons, solvents, pesticides, lead, and other heavy metals. Contamination is correlated with the degree of industrialization and intensity of chemical substance. The concern over soil contamination stems primarily from health risks, from direct contact with the contaminated soil, vapour from the contaminants, or from secondary contamination of water supplies within and underlying the soil. Mapping of contaminated soil sites and the resulting clean ups are time-consuming and expensive tasks, and require expertise in geology, hydrology, chemistry, computer modelling, and GIS in Environmental Contamination, as well as an appreciation of the history of industrial chemistry.
Zinc toxicity is a medical condition involving an overdose on, or toxic overexposure to, zinc. Such toxicity levels have been seen to occur at ingestion of greater than 50 mg of zinc. Excessive absorption of zinc can suppress copper and iron absorption. The free zinc ion in solution is highly toxic to bacteria, plants, invertebrates, and even vertebrate fish. Zinc is an essential trace metal with very low toxicity in humans.

Cylindrospermopsin is a cyanotoxin produced by a variety of freshwater cyanobacteria. CYN is a polycyclic uracil derivative containing guanidino and sulfate groups. It is also zwitterionic, making it highly water soluble. CYN is toxic to liver and kidney tissue and is thought to inhibit protein synthesis and to covalently modify DNA and/or RNA. It is not known whether cylindrospermopsin is a carcinogen, but it appears to have no tumour initiating activity in mice.

Environmental toxicology is a multidisciplinary field of science concerned with the study of the harmful effects of various chemical, biological and physical agents on living organisms. Ecotoxicology is a subdiscipline of environmental toxicology concerned with studying the harmful effects of toxicants at the population and ecosystem levels.
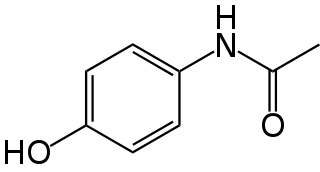
Paracetamol poisoning, also known as acetaminophen poisoning, is caused by excessive use of the medication paracetamol (acetaminophen). Most people have few or non-specific symptoms in the first 24 hours following overdose. These symptoms include feeling tired, abdominal pain, or nausea. This is typically followed by absence of symptoms for a couple of days, after which yellowish skin, blood clotting problems, and confusion occurs as a result of liver failure. Additional complications may include kidney failure, pancreatitis, low blood sugar, and lactic acidosis. If death does not occur, people tend to recover fully over a couple of weeks. Without treatment, death from toxicity occurs 4 to 18 days later.

Senecionine is a toxic pyrrolizidine alkaloid isolated from various botanical sources. It takes its name from the Senecio genus and is produced by many different plants in that genus, including Jacobaea vulgaris. It has also been isolated from several other plants, including Brachyglottis repanda, Emilia, Erechtites hieraciifolius, Petasites, Syneilesis, Crotalaria, Caltha leptosepala, and Castilleja.
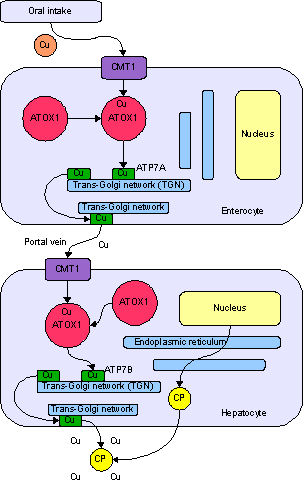
Copper is an essential trace element that is vital to the health of all living things. In humans, copper is essential to the proper functioning of organs and metabolic processes. The human body has complex homeostatic mechanisms which attempt to ensure a constant supply of available copper, while eliminating excess copper whenever this occurs. However, like all essential elements and nutrients, too much or too little nutritional ingestion of copper can result in a corresponding condition of copper excess or deficiency in the body, each of which has its own unique set of adverse health effects.
















