Related Research Articles

The Cretaceous is a geological period that lasted from about 145 to 66 million years ago (Mya). It is the third and final period of the Mesozoic Era, as well as the longest. At around 79 million years, it is the longest geological period of the entire Phanerozoic. The name is derived from the Latin creta, "chalk", which is abundant in the latter half of the period. It is usually abbreviated K, for its German translation Kreide.

The Cenozoic is Earth's current geological era, representing the last 66 million years of Earth's history. It is characterised by the dominance of mammals, birds, and angiosperms. It is the latest of three geological eras, preceded by the Mesozoic and Paleozoic. The Cenozoic started with the Cretaceous–Paleogene extinction event, when many species, including the non-avian dinosaurs, became extinct in an event attributed by most experts to the impact of a large asteroid or other celestial body, the Chicxulub impactor.

The Jurassic is a geologic period and stratigraphic system that spanned from the end of the Triassic Period 201.4 million years ago (Mya) to the beginning of the Cretaceous Period, approximately 145 Mya. The Jurassic constitutes the middle period of the Mesozoic Era and is named after the Jura Mountains, where limestone strata from the period were first identified.

Flowering plants are plants that bear flowers and fruits, and form the clade Angiospermae, commonly called angiosperms. They include all forbs, grasses and grass-like plants, a vast majority of broad-leaved trees, shrubs and vines, and most aquatic plants. The term "angiosperm" is derived from the Greek words ἀγγεῖον / angeion and σπέρμα / sperma ('seed'), meaning that the seeds are enclosed within a fruit. They are by far the most diverse group of land plants with 64 orders, 416 families, approximately 13,000 known genera and 300,000 known species. Angiosperms were formerly called Magnoliophyta.
The Mesozoic Era is the second-to-last era of Earth's geological history, lasting from about 252 to 66 million years ago, comprising the Triassic, Jurassic and Cretaceous Periods. It is characterized by the dominance of gymnosperms and of archosaurian reptiles, such as the dinosaurs; a hot greenhouse climate; and the tectonic break-up of Pangaea. The Mesozoic is the middle of the three eras since complex life evolved: the Paleozoic, the Mesozoic, and the Cenozoic.
The Phanerozoic is the current and the latest of the four geologic eons in the Earth's geologic time scale, covering the time period from 538.8 million years ago to the present. It is the eon during which abundant animal and plant life has proliferated, diversified and colonized various niches on the Earth's surface, beginning with the Cambrian period when animals first developed hard shells that can be clearly preserved in the fossil record. The time before the Phanerozoic, collectively called the Precambrian, is now divided into the Hadean, Archaean and Proterozoic eons.

The Zingiberales are flowering plants forming one of four orders in the commelinids clade of monocots, together with its sister order, Commelinales. The order includes 68 genera and 2,600 species. Zingiberales are a unique though morphologically diverse order that has been widely recognised as such over a long period of time. They are usually large herbaceous plants with rhizomatous root systems and lacking an aerial stem except when flowering. Flowers are usually large and showy, and the stamens are often modified (staminodes) to also form colourful petal-like structures that attract pollinators.

Cycads are seed plants that typically have a stout and woody (ligneous) trunk with a crown of large, hard, stiff, evergreen and (usually) pinnate leaves. The species are dioecious, that is, individual plants of a species are either male or female. Cycads vary in size from having trunks only a few centimeters to several meters tall. They typically grow very slowly and live very long. Because of their superficial resemblance, they are sometimes mistaken for palms or ferns, but they are not closely related to either group.

Gnetophyta is a division of plants, grouped within the gymnosperms, that consists of some 70 species across the three relict genera: Gnetum, Welwitschia, and Ephedra. The earliest unambiguous records of the group date to the Jurassic, and they achieved their highest diversity during the Early Cretaceous. The primary difference between gnetophytes and other gymnosperms is the presence of vessel elements, a system of small tubes (xylem) that transport water within the plant, similar to those found in flowering plants. Because of this, gnetophytes were once thought to be the closest gymnosperm relatives to flowering plants, but more recent molecular studies have brought this hypothesis into question, with many recent phylogenies finding them to be nested within the conifers.

The gymnosperms are a group of seed-producing plants that includes conifers, cycads, Ginkgo, and gnetophytes, forming the clade Gymnospermae. The term gymnosperm comes from the composite word in Greek: γυμνόσπερμος, literally meaning 'naked seeds'. The name is based on the unenclosed condition of their seeds. The non-encased condition of their seeds contrasts with the seeds and ovules of flowering plants (angiosperms), which are enclosed within an ovary. Gymnosperm seeds develop either on the surface of scales or leaves, which are often modified to form cones, or on their own as in yew, Torreya, Ginkgo. Gymnosperm lifecycles involve alternation of generations. They have a dominant diploid sporophyte phase and a reduced haploid gametophyte phase which is dependent on the sporophytic phase. The term "gymnosperm" is often used in paleobotany to refer to all non-angiosperm seed plants. In that case, to specify the modern monophyletic group of gymnosperms, the term Acrogymnospermae is sometimes used.

Bennettitales is an extinct order of seed plants that first appeared in the Permian period and became extinct in most areas toward the end of the Cretaceous. Bennettitales were amongst the most common seed plants of the Mesozoic, and had morphologies including shrub and cycad-like forms. The foliage of bennettitaleans is superficially nearly indistinguishable from that of cycads, but they are distinguished from cycads by their more complex flower-like reproductive organs, at least some of which were likely pollinated by insects.

Entomophily or insect pollination is a form of pollination whereby pollen of plants, especially but not only of flowering plants, is distributed by insects. Flowers pollinated by insects typically advertise themselves with bright colours, sometimes with conspicuous patterns leading to rewards of pollen and nectar; they may also have an attractive scent which in some cases mimics insect pheromones. Insect pollinators such as bees have adaptations for their role, such as lapping or sucking mouthparts to take in nectar, and in some species also pollen baskets on their hind legs. This required the coevolution of insects and flowering plants in the development of pollination behaviour by the insects and pollination mechanisms by the flowers, benefiting both groups. Both the size and the density of a population are known to affect pollination and subsequent reproductive performance.

The evolution of plants has resulted in a wide range of complexity, from the earliest algal mats of unicellular archaeplastids evolved through endosymbiosis, through multicellular marine and freshwater green algae, to spore-bearing terrestrial bryophytes, lycopods and ferns, and eventually to the complex seed-bearing gymnosperms and angiosperms of today. While many of the earliest groups continue to thrive, as exemplified by red and green algae in marine environments, more recently derived groups have displaced previously ecologically dominant ones; for example, the ascendance of flowering plants over gymnosperms in terrestrial environments.
The natural history of New Zealand began when the landmass Zealandia broke away from the supercontinent Gondwana in the Cretaceous period. Before this time, Zealandia shared its past with Australia and Antarctica. Since this separation, the New Zealand landscape has evolved in physical isolation, although much of its current biota has more recent connections with species on other landmasses. The exclusively natural history of the country ended in about 1300 AD, when humans first settled, and the country's environmental history began. The period from 1300 AD to today coincides with the extinction of many of New Zealand's unique species that had evolved there.
The Paleocene, or Palaeocene, is a geological epoch that lasted from about 66 to 56 million years ago (mya). It is the first epoch of the Paleogene Period in the modern Cenozoic Era. The name is a combination of the Ancient Greek παλαιός palaiós meaning "old" and the Eocene Epoch, translating to "the old part of the Eocene".
Cretaceous polar forests were temperate forests that grew at polar latitudes during the final period of the Mesozoic Era, known as the Cretaceous Period 145–66 Ma. During this period, global average temperature was about 10 °C (18 °F) higher and carbon dioxide (CO2) levels were approximately 1000 parts per million (ppm), 2.5 times the current concentration in Earth's atmosphere. The abundance of atmospheric carbon dioxide had a very significant impact on global climate and Earth's natural systems as its concentration is considered one of the main factors in the development of a pronounced greenhouse Earth during the Cretaceous, with a very low average global temperature gradient. As a consequence, high paleolatitudes in both hemispheres were much warmer than at present. This temperature gradient was partly responsible for the lack of continental ice sheets in polar regions.
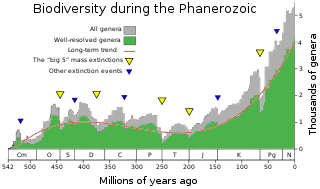
The Mesozoic–Cenozoic Radiation is the third major extended increase of biodiversity in the Phanerozoic, after the Cambrian Explosion and the Great Ordovician Biodiversification Event, which appeared to exceeded the equilibrium reached after the Ordovician radiation. Made known by its identification in marine invertebrates, this evolutionary radiation began in the Mesozoic, after the Permian extinctions, and continues to this date. This spectacular radiation affected both terrestrial and marine flora and fauna, during which the "modern" fauna came to replace much of the Paleozoic fauna. Notably, this radiation event was marked by the rise of angiosperms during the mid-Cretaceous, and the K-Pg extinction, which initiated the rapid increase in mammalian biodiversity.

The Cretaceous–Paleogene (K–Pg) extinction event, also known as the Cretaceous–Tertiary(K–T)extinction, was a sudden mass extinction of three-quarters of the plant and animal species on Earth, approximately 66 million years ago. The event caused the extinction of all non-avian dinosaurs. Most other tetrapods weighing more than 25 kilograms also became extinct, with the exception of some ectothermic species such as sea turtles and crocodilians. It marked the end of the Cretaceous period, and with it the Mesozoic era, while heralding the beginning of the current era, the Cenozoic. In the geologic record, the K–Pg event is marked by a thin layer of sediment called the K–Pg boundary or K–T boundary, which can be found throughout the world in marine and terrestrial rocks. The boundary clay shows unusually high levels of the metal iridium, which is more common in asteroids than in the Earth's crust.
This article records new taxa of fossil plants that are scheduled to be described during the year 2021, as well as other significant discoveries and events related to paleobotany that are scheduled to occur in the year 2021.
The fossil history of flowering plants records the development of flowers and other distinctive structures of the angiosperms, now the dominant group of plants on land. The history is controversial as flowering plants appear in great diversity in the Cretaceous, with scanty and debatable records before that, creating a puzzle for evolutionary biologists that Charles Darwin named an "abominable mystery". Nonetheless, in April 2024, scientists reported an overview of the origin and development of flowering plants over the years based on extensive genetic studies.
References
- 1 2 Benton, Michael James; Wilf, Peter; Sauquet, Hervé (26 October 2021). "The Angiosperm Terrestrial Revolution and the origins of modern biodiversity". New Phytologist . 233 (5): 2017–2035. doi:10.1111/nph.17822. hdl: 1983/82a09075-31f4-423e-98b9-3bb2c215e04b . PMID 34699613. S2CID 240000207 . Retrieved 24 November 2022.
- 1 2 Lloyd, G. T.; et al. (2008). "Dinosaurs and the Cretaceous Terrestrial Revolution. 2008". Proceedings of the Royal Society B: Biological Sciences . 275 (1650): 2483–2490. doi:10.1098/rspb.2008.0715. PMC 2603200 . PMID 18647715.
- ↑ Barba-Montoya, Jose; Dos Reis, Mario; Schneider, Harald; Donoghue, Philip C. J.; Yang, Ziheng (5 February 2018). "Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a Cretaceous Terrestrial Revolution". New Phytologist . 218 (2): 819–834. doi:10.1111/nph.15011. PMC 6055841 . PMID 29399804.
- ↑ Gurung, Khushboo; Field, Katie J.; Batterman, Sarah J.; Goddéris, Yves; Donnadieu, Yannick; Porada, Philipp; Taylor, Lyla L.; Mills, Benjamin J. W. (4 August 2022). "Climate windows of opportunity for plant expansion during the Phanerozoic". Nature Communications . 13 (1): 4530. Bibcode:2022NatCo..13.4530G. doi:10.1038/s41467-022-32077-7. PMC 9352767 . PMID 35927259.
- ↑ de Boer, Hugo Jan; Eppinga, Maarten B.; Wassen, Martin J.; Dekker, Stefan C. (27 November 2012). "A critical transition in leaf evolution facilitated the Cretaceous angiosperm revolution". Nature Communications . 3 (1): 1221. Bibcode:2012NatCo...3.1221D. doi:10.1038/ncomms2217. ISSN 2041-1723. PMC 3514505 . PMID 23187621.
- ↑ Boyce, C. Kevin; Zwieniecki, Maciej A. (26 June 2012). "Leaf fossil record suggests limited influence of atmospheric CO 2 on terrestrial productivity prior to angiosperm evolution". Proceedings of the National Academy of Sciences of the United States of America . 109 (26): 10403–10408. doi: 10.1073/pnas.1203769109 . ISSN 0027-8424. PMC 3387114 . PMID 22689947.
- ↑ Friis, E.M.; Pedersen, K. Raunsgaard; Crane, P.R. (22 March 2006). "Cretaceous angiosperm flowers: Innovation and evolution in plant reproduction". Palaeogeography, Palaeoclimatology, Palaeoecology . 232 (2–4): 251–293. Bibcode:2006PPP...232..251F. doi:10.1016/j.palaeo.2005.07.006 . Retrieved 20 May 2024– via Elsevier Science Direct.
- ↑ Meredith, Robert W. (2011). "Impacts of the Cretaceous Terrestrial Revolution and KPg Extinction on Mammal Diversification". Science . 334 (6055): 521–524. Bibcode:2011Sci...334..521M. doi:10.1126/science.1211028. PMID 21940861. S2CID 38120449.
- ↑ Grossnickle, David M.; Polly, P. David (22 November 2013). "Mammal disparity decreases during the Cretaceous angiosperm radiation". Proceedings of the Royal Society B: Biological Sciences . 280 (1771): 20132110. doi:10.1098/rspb.2013.2110. ISSN 0962-8452. PMC 3790494 . PMID 24089340.
- ↑ Tihelka, Erik; Cai, Chenyang; Giacomelli, Mattia; Pisani, Davide; Donoghue, Philip C. J. (11 November 2020). "Integrated phylogenomic and fossil evidence of stick and leaf insects (Phasmatodea) reveal a Permian–Triassic co-origination with insectivores". Royal Society Open Science . 7 (11): 201689. Bibcode:2020RSOS....701689T. doi:10.1098/rsos.201689. PMC 7735357 . PMID 33391817.
- ↑ Jouault, Corentin; Condamine, Fabien L.; Legendre, Frédéric; Perrichot, Vincent (11 March 2024). "The Angiosperm Terrestrial Revolution buffered ants against extinction". Proceedings of the National Academy of Sciences of the United States of America . 121 (13): e2317795121. Bibcode:2024PNAS..12117795J. doi:10.1073/pnas.2317795121. ISSN 0027-8424. PMC 10990090. PMID 38466878 . Retrieved 16 March 2024.
- ↑ Cardinal, S.; Straka, J.; Danforth, B. N. (2010). "Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism". Proceedings of the National Academy of Sciences of the United States of America . 107 (37): 16207–11. Bibcode:2010PNAS..10716207C. doi: 10.1073/pnas.1006299107 . PMC 2941306 . PMID 20805492.
- ↑ Peñalver, Enrique; Arillo, Antonio; Pérez-de la Fuente, Ricardo; Riccio, Mark L.; Delclòs, Xavier; Barrón, Eduardo; Grimaldi, David A. (9 July 2015). "Long-Proboscid Flies as Pollinators of Cretaceous Gymnosperms". Current Biology . 25 (14): 1917–1923. Bibcode:2015CBio...25.1917P. doi:10.1016/j.cub.2015.05.062. PMID 26166781 . Retrieved 20 May 2024.
- ↑ Zhang, Qingqing; Wang, Bo (24 April 2017). "Evolution of Lower Brachyceran Flies (Diptera) and Their Adaptive Radiation with Angiosperms". Frontiers in Plant Science . 8: 631. doi: 10.3389/fpls.2017.00631 . ISSN 1664-462X. PMC 5401883 . PMID 28484485.
- ↑ Bao, Tong; Wang, Bo; Li, Jianguo; Dilcher, David (3 December 2019). "Pollination of Cretaceous flowers". Proceedings of the National Academy of Sciences of the United States of America . 116 (49): 24707–24711. Bibcode:2019PNAS..11624707B. doi: 10.1073/pnas.1916186116 . ISSN 0027-8424. PMC 6900596 . PMID 31712419.
- ↑ Tihelka, Erik; Li, Liqin; Fu, Yanzhe; Su, Yitong; Huang, Diying; Cai, Chenyang (12 April 2021). "Angiosperm pollinivory in a Cretaceous beetle". Nature Plants . 7 (4): 445–451. doi:10.1038/s41477-021-00893-2. ISSN 2055-0278. PMID 33846595 . Retrieved 20 May 2024.
- ↑ Pellmyr, Olle (February 1992). "Evolution of insect pollination and angiosperm diversification". Trends in Ecology & Evolution . 7 (2): 46–49. doi:10.1016/0169-5347(92)90105-K. PMID 21235949 . Retrieved 20 May 2024.
- ↑ Schneider, Harald (28 January 2016). "The ghost of the Cretaceous terrestrial revolution in the evolution of fern–sawfly associations". Journal of Systematics and Evolution . 54 (2): 93–103. doi:10.1111/jse.12194. ISSN 1674-4918 . Retrieved 11 May 2024– via Wiley Online Library.
- ↑ Li, Xin-Ran; Huang, Di-Ying (29 March 2023). "Atypical 'long-tailed' cockroaches arose during Cretaceous in response to angiosperm terrestrial revolution". PeerJ . 11: e15067. doi: 10.7717/peerj.15067 . ISSN 2167-8359. PMC 10066690 . PMID 37013144.
- ↑ Boyce, C. Kevin; Lee, Jung-Eun (1 June 2011). "Could Land Plant Evolution Have Fed the Marine Revolution?". Paleontological Research. 15 (2): 100–105. doi:10.2517/1342-8144-15.2.100. ISSN 1342-8144 . Retrieved 29 September 2023.
- ↑ Vermeij, Geerat J.; Grosberg, Richard K. (2 July 2010). "The Great Divergence: When Did Diversity on Land Exceed That in the Sea?". Integrative and Comparative Biology . 50 (4): 675–682. doi: 10.1093/icb/icq078 . PMID 21558232 . Retrieved 1 October 2022.