Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. Actinium gave the name to the actinide series, a set of 15 elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.

Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an atomic number that is reduced by two. An alpha particle is identical to the nucleus of a helium-4 atom, which consists of two protons and two neutrons. It has a charge of +2 e and a mass of 4 Da. For example, uranium-238 decays to form thorium-234.

Polonium is a chemical element with the symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though longer-lived isotopes exist, such as the 125.2 years half-life of polonium 209, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.

Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1,600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence.

Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a treatment using ionizing radiation, generally provided as part of cancer therapy to either kill or control the growth of malignant cells. It is normally delivered by a linear particle accelerator. Radiation therapy may be curative in a number of types of cancer if they are localized to one area of the body, and have not spread to other parts. It may also be used as part of adjuvant therapy, to prevent tumor recurrence after surgery to remove a primary malignant tumor. Radiation therapy is synergistic with chemotherapy, and has been used before, during, and after chemotherapy in susceptible cancers. The subspecialty of oncology concerned with radiotherapy is called radiation oncology. A physician who practices in this subspecialty is a radiation oncologist.
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.

Radioactive decay is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha, beta, and gamma decay, all of which involve emitting particles. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force.
Radionuclide therapy uses radioactive substances called radiopharmaceuticals to treat medical conditions, particularly cancer. These are introduced into the body by various means and localise to specific locations, organs or tissues depending on their properties and administration routes. This includes anything from a simple compound such as sodium iodide that locates to the thyroid via trapping the iodide ion, to complex biopharmaceuticals such as recombinant antibodies which are attached to radionuclides and seek out specific antigens on cell surfaces.

The Geiger–Marsden experiments were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1908 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester.
Polonium-210 is an isotope of polonium. It undergoes alpha decay to stable 206Pb with a half-life of 138.376 days, the longest half-life of all naturally occurring polonium isotopes. First identified in 1898, and also marking the discovery of the element polonium, 210Po is generated in the decay chain of uranium-238 and radium-226. 210Po is a prominent contaminant in the environment, mostly affecting seafood and tobacco. Its extreme toxicity is attributed to intense radioactivity, capable of severely harming humans.

Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell. It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells.
Naturally occurring radioactive materials (NORM) and technologically enhanced naturally occurring radioactive materials (TENORM) consist of materials, usually industrial wastes or by-products enriched with radioactive elements found in the environment, such as uranium, thorium and potassium and any of their decay products, such as radium and radon. Produced water discharges and spills are a good example of entering NORMs into the surrounding environment.
Radiobiology is a field of clinical and basic medical sciences that involves the study of the effects of ionizing radiation on living things, in particular health effects of radiation. Ionizing radiation is generally harmful and potentially lethal to living things but can have health benefits in radiation therapy for the treatment of cancer and thyrotoxicosis. Its most common impact is the induction of cancer with a latent period of years or decades after exposure. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Controlled doses are used for medical imaging and radiotherapy.

A gamma ray, also known as gamma radiation (symbol γ or ), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (3×1019 Hz), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.

Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, α. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+
or 4
2He2+
indicating a helium ion with a +2 charge. Once the ion gains electrons from its environment, the alpha particle becomes a normal helium atom 4
2He.
Radium-223 is an isotope of radium with an 11.4-day half-life. It was discovered in 1905 by T. Godlewski, a Polish chemist from Kraków, and was historically known as actinium X (AcX). Radium-223 dichloride is an alpha particle-emitting radiotherapy drug that mimics calcium and forms complexes with hydroxyapatite at areas of increased bone turnover. The principal use of radium-223, as a radiopharmaceutical to treat metastatic cancers in bone, takes advantage of its chemical similarity to calcium, and the short range of the alpha radiation it emits.
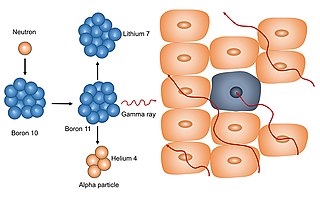
Neutron capture therapy (NCT) is a type of radiotherapy for treating locally invasive malignant tumors such as primary brain tumors, recurrent cancers of the head and neck region, and cutaneous and extracutaneous melanomas. It is a two-step process: first, the patient is injected with a tumor-localizing drug containing the stable isotope boron-10 (10B), which has a high propensity to capture low energy "thermal" neutrons. The neutron cross section of 10B is 1,000 times more than that of other elements, such as nitrogen, hydrogen, or oxygen, that occur in tissue. In the second step, the patient is radiated with epithermal neutrons, the sources of which in the past have been nuclear reactors and now are accelerators that produce higher energy epithermal neutrons. After losing energy as they penetrate tissue, the resultant low energy "thermal" neutrons are captured by the 10B atoms. The resulting decay reaction yields high-energy alpha particles that kill the cancer cells that have taken up enough 10B.
Targeted alpha-particle therapy is an in-development method of targeted radionuclide therapy of various cancers. It employs radioactive substances which undergo alpha decay to treat diseased tissue at close proximity. It has the potential to provide highly targeted treatment, especially to microscopic tumour cells. Targets include leukemias, lymphomas, gliomas, melanoma, and peritoneal carcinomatosis. As in diagnostic nuclear medicine, appropriate radionuclides can be chemically bound to a targeting biomolecule which carries the combined radiopharmaceutical to a specific treatment point.
Combinatorial ablation and immunotherapy is an oncological treatment that combines various tumor-ablation techniques with immunotherapy treatment. Combining ablation therapy of tumors with immunotherapy enhances the immunostimulating response and has synergistic effects for curative metastatic cancer treatment. Various ablative techniques are utilized including cryoablation, radiofrequency ablation, laser ablation, photodynamic ablation, stereotactic radiation therapy, alpha-emitting radiation therapy, hyperthermia therapy, HIFU. Thus, combinatorial ablation of tumors and immunotherapy is a way of achieving an autologous, in-vivo tumor lysate vaccine and treating metastatic disease.

Peptide receptor radionuclide therapy (PRRT) is a type of radionuclide therapy, using a radiopharmaceutical that targets peptide receptors to deliver localised treatment, typically for neuroendocrine tumours (NETs).









