
In pharmacology and toxicology, a route of administration is the way by which a drug, fluid, poison, or other substance is taken into the body.
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; the terms are equivalent in the literature. Larger structures such as nucleic acids and proteins, and many polysaccharides are not small molecules, although their constituent monomers are often considered small molecules. Small molecules may be used as research tools to probe biological function as well as leads in the development of new therapeutic agents. Some can inhibit a specific function of a protein or disrupt protein–protein interactions.
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.

ADME is the four-letter abbreviation (acronym) for absorption, distribution, metabolism, and excretion, and is mainly used in fields such as pharmacokinetics and pharmacology. The four letter stands for descriptors quantifying how a given drug interacts within body over time. The term ADME was first introduced in the 1960s, and has become a standard term widely used in scientific literature, teaching, drug regulations, and clinical practice.

Physiologically based pharmacokinetic (PBPK) modeling is a mathematical modeling technique for predicting the absorption, distribution, metabolism and excretion (ADME) of synthetic or natural chemical substances in humans and other animal species. PBPK modeling is used in pharmaceutical research and drug development, and in health risk assessment for cosmetics or general chemicals.

Caco-2 is an immortalized cell line of human colorectal adenocarcinoma cells. It is primarily used as a model of the intestinal epithelial barrier. In culture, Caco-2 cells spontaneously differentiate into a heterogeneous mixture of intestinal epithelial cells. It was developed in 1977 by Jorgen Fogh at the Sloan-Kettering Institute for Cancer Research.
The Biopharmaceutics Classification System (BCS) is a system to differentiate drugs on the basis of their solubility and permeability.

The polar surface area (PSA) or topological polar surface area (TPSA) of a molecule is defined as the surface sum over all polar atoms or molecules, primarily oxygen and nitrogen, also including their attached hydrogen atoms.
Absorption is the journey of a drug travelling from the site of administration to the site of action.
Paracellular transport refers to the transfer of substances across an epithelium by passing through the intercellular space between the cells. It is in contrast to transcellular transport, where the substances travel through the cell, passing through both the apical membrane and basolateral membrane.
A self-microemulsifying drug delivery system (SMEDDS) is a drug delivery system that uses a microemulsion achieved by chemical rather than mechanical means. That is, by an intrinsic property of the drug formulation, rather than by special mixing and handling. It employs the familiar ouzo effect displayed by anethole in many anise-flavored liquors. Microemulsions have significant potential for use in drug delivery, and SMEDDS are the best of these systems identified to date. SMEDDS are of particular value in increasing the absorption of lipophilic drugs taken by mouth.

Lipid nanoparticles (LNPs) are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro. LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle.
Transepithelial potential difference (TEPD) is the voltage across an epithelium, and is the sum of the membrane potentials for the outer and inner cell membranes.
In medicinal chemistry, parallel artificial membrane permeability assay (PAMPA) is a method which determines the permeability of substances from a donor compartment, through a lipid-infused artificial membrane into an acceptor compartment. A multi-well microtitre plate is used for the donor and a membrane/acceptor compartment is placed on top; the whole assembly is commonly referred to as a “sandwich”. At the beginning of the test, the drug is added to the donor compartment, and the acceptor compartment is drug-free. After an incubation period which may include stirring, the sandwich is separated and the amount of drug is measured in each compartment. Mass balance allows calculation of drug that remains in the membrane.
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture, integrated circuit (chip) that simulates the activities, mechanics and physiological response of an entire organ or an organ system. It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context. By acting as a more sophisticated in vitro approximation of complex tissues than standard cell culture, they provide the potential as an alternative to animal models for drug development and toxin testing.
Buccal administration is a topical route of administration by which drugs held or applied in the buccal area diffuse through the oral mucosa and enter directly into the bloodstream. Buccal administration may provide better bioavailability of some drugs and a more rapid onset of action compared to oral administration because the medication does not pass through the digestive system and thereby avoids first pass metabolism. Drug forms for buccal administration include tablets and thin films.
Topical drug delivery (TDD) is a route of drug administration that allows the topical formulation to be delivered across the skin upon application, hence producing a localized effect to treat skin disorders like eczema. The formulation of topical drugs can be classified into corticosteroids, antibiotics, antiseptics, and anti-fungal. The mechanism of topical delivery includes the diffusion and metabolism of drugs in the skin. Historically, topical route was the first route of medication used to deliver drugs in humans in ancient Egyptian and Babylonian in 3000 BCE. In these ancient cities, topical medications like ointments and potions were used on the skin. The delivery of topical drugs needs to pass through multiple skin layers and undergo pharmacokinetics, hence factor like dermal diseases minimize the bioavailability of the topical drugs. The wide use of topical drugs leads to the advancement in topical drug delivery. These advancements are used to enhance the delivery of topical medications to the skin by using chemical and physical agents. For chemical agents, carriers like liposomes and nanotechnologies are used to enhance the absorption of topical drugs. On the other hand, physical agents, like micro-needles is other approach for enhancement ofabsorption. Besides using carriers, other factors such as pH, lipophilicity, and drug molecule size govern the effectiveness of topical formulation.
Penetration enhancers are chemical compounds that can facilitate the penetration of active pharmaceutical ingredients (API) into or through the poorly permeable biological membranes. These compounds are used in some pharmaceutical formulations to enhance the penetration of APIs in transdermal drug delivery and transmucosal drug delivery. They typically penetrate into the biological membranes and reversibly decrease their barrier properties.
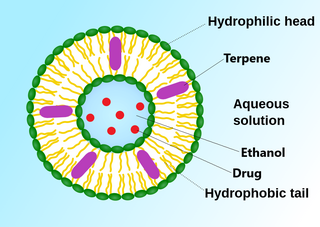
An invasome are a type of artificial vesicle nanocarrier that transport substances through the skin, the most superficial biological barrier. Vesicles are small particles surrounded by a lipid layer that can carry substances into and out of the cell. Artificial vesicles can be engineered to deliver drugs within the cell, with specific applications within transdermal drug delivery. However, the skin proves to be a barrier to effective penetration and delivery of drug therapies. Thus, invasomes are a new generation of vesicle with added structural components to assist with skin penetration.
Klara Valko is a scientist, consultant, academic and author. She is the director of Bio-Mimetic Chromatography as well as an honorary professor at University College London School of Pharmacy.









