
Soman is an extremely toxic chemical substance. It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiting the enzyme cholinesterase. It is an inhibitor of both acetylcholinesterase and butyrylcholinesterase. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687. Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance. Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin).

Parathion, also called parathion-ethyl or diethyl parathion and locally known as "Folidol", is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. The basic structure is shared by parathion methyl.

Chlorfenvinphos is an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was cancelled in 1991.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. Ethion is known to affect a neural enzyme called acetylcholinesterase and prevent it from working.

Diazinon, a colorless to dark brown liquid, is a thiophosphoric acid ester developed in 1952 by Ciba-Geigy, a Swiss chemical company. It is a nonsystemic organophosphate insecticide formerly used to control cockroaches, silverfish, ants, and fleas in residential, non-food buildings. Diazinon was heavily used during the 1970s and early 1980s for general-purpose gardening use and indoor pest control. A bait form was used to control scavenger wasps in the western U.S. Diazinon is used in flea collars for domestic pets in Australia and New Zealand. Residential uses of diazinon were outlawed in the U.S. in 2004 because of human health risks but it is still approved for agricultural uses. An emergency antidote is atropine.
Demeton-S-methyl is an organic compound with the molecular formula C6H15O3PS2. It was used as an organothiophosphate acaricide and organothiophosphate insecticide. It is flammable. With prolonged storage, Demeton-S-methyl becomes more toxic due to formation of a sulfonium derivative which has greater affinity to the human form of the acetylcholinesterase enzyme, and this may present a hazard in agricultural use.

p-Xylene (para-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The p- stands for para-, indicating that the two methyl groups in p-xylene occupy the diametrically opposite substituent positions 1 and 4. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and m-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable. The odor threshold of p-xylene is 0.62 parts per million (ppm).

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.

Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.

Dimethyl phthalate (DMP) is an organic compound and phthalate ester. it is a colourless and oily liquid that is soluble in organic solvents, but which is only poorly soluble in water.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

α-Naphthylthiourea (ANTU) is an organosulfur compound with the formula C10H7NHC(S)NH2. This a white, crystalline powder although commercial samples may be off-white. It is used as a rodenticide and as such is fairly toxic. Naphthylthiourea is available as 10% active baits in suitable protein- or carbohydrate-rich materials and as a 20% tracking powder.
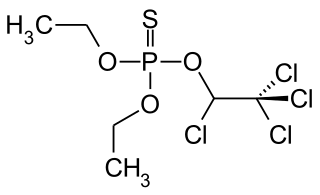
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.

Disulfoton is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is manufactured under the name Di-Syston by Bayer CropScience. Disulfoton in its pure form is a colorless oil but the technical product used in vegetable fields is dark and yellowish with a sulfur odor. Disulfoton is processed as a liquid into carrier granules, these granules are mixed with fertilizer and clay to be made into a spike, designed to be driven into the ground. The pesticide is absorbed over time by the roots and translocated to all parts of the plant. The pesticide acts as a cholinesterase inhibitor and gives long lasting control.

Demeton, sold as an amber oily liquid with a sulphur like odour under the name Systox, is an organophosphate derivative causing irritability and shortness of breath to individuals repeatedly exposed. It was used as a phosphorothioate insecticide and acaricide and has the chemical formula C8H19O3PS2. Although it was previously used as an insecticide, it is now largely obsolete due to its relatively high toxicity to humans. Demeton consists of two components, demeton-S and demeton-O in a ratio of approximately 2:1 respectively. The chemical structure of demeton is closely related to military nerve agents such as VX and a derivative with one of the ethoxy groups replaced by methyl was investigated by both the US and Soviet chemical-weapons programs under the names V.sub.X and GD-7.

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.

Parathion methyl, or methyl parathion, is an organophosphate insecticide, possessing an organothiophosphate group. It is structurally very similar to parathion-ethyl. It is not allowed for sale and import in nearly all countries around the world, while a few allow it under subject to specified conditions only.
Butyl acrylate is an organic compound with the formula C4H9O2CCH=CH2. A colorless liquid, it is the butyl ester of acrylic acid. It is used commercially on a large scale as a precursor to poly(butyl acrylate). Especially as copolymers, such materials are used in paints, sealants, coatings, adhesives, fuel, textiles, plastics, and caulk.

EA-3990 is a deadly carbamate nerve agent. It is lethal because it inhibits acetylcholinesterase. Inhibition causes an overly high accumulation of acetylcholine between the nerve and muscle cells. This paralyzes the muscles by preventing their relaxation. The paralyzed muscles include the muscles used for breathing.

EA-4056 is a deadly carbamate nerve agent. It is lethal because it inhibits acetylcholinesterase. Inhibition causes an overly high accumulation of acetylcholine between the nerve and muscle cells. This paralyzes the muscles by preventing their relaxation. The paralyzed muscles includes the muscles used for breathing.


















