
Epilepsy is a group of non-communicable neurological disorders characterized by recurrent epileptic seizures. An epileptic seizure is the clinical manifestation of an abnormal, excessive, and synchronized electrical discharge in the neurons. The occurrence of two or more unprovoked seizures defines epilepsy. The occurrence of just one seizure may warrant the definition in a more clinical usage where recurrence may be able to be prejudged. Epileptic seizures can vary from brief and nearly undetectable periods to long periods of vigorous shaking due to abnormal electrical activity in the brain. These episodes can result in physical injuries, either directly such as broken bones or through causing accidents. In epilepsy, seizures tend to recur and may have no detectable underlying cause. Isolated seizures that are provoked by a specific cause such as poisoning are not deemed to represent epilepsy. People with epilepsy may be treated differently in various areas of the world and experience varying degrees of social stigma due to the alarming nature of their symptoms.

Phenytoin (PHT), sold under the brand name Dilantin among others, is an anti-seizure medication. It is useful for the prevention of tonic-clonic seizures and focal seizures, but not absence seizures. The intravenous form, fosphenytoin, is used for status epilepticus that does not improve with benzodiazepines. It may also be used for certain heart arrhythmias or neuropathic pain. It can be taken intravenously or by mouth. The intravenous form generally begins working within 30 minutes and is effective for roughly 24 hours. Blood levels can be measured to determine the proper dose.

Valproate are medications primarily used to treat epilepsy and bipolar disorder and prevent migraine headaches. They are useful for the prevention of seizures in those with absence seizures, partial seizures, and generalized seizures. They can be given intravenously or by mouth, and the tablet forms exist in both long- and short-acting formulations.
Anticonvulsants are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain.
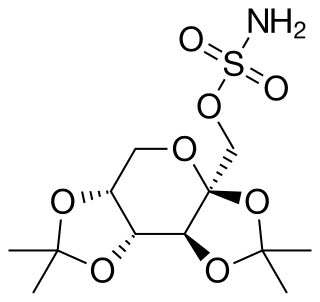
Topiramate, sold under the brand name Topamax among others, is a medication used to treat epilepsy and prevent migraines. It has also been used in alcohol dependence and essential tremor. For epilepsy this includes treatment for generalized or focal seizures. It is taken orally.

Lamotrigine, sold under the brand name Lamictal among others, is a medication used to treat epilepsy and stabilize mood in bipolar disorder. For epilepsy, this includes focal seizures, tonic-clonic seizures, and seizures in Lennox-Gastaut syndrome. In bipolar disorder, lamotrigine has not been shown to reliably treat acute depression for all groups except in the severely depressed; but for patients with bipolar disorder who are not currently symptomatic, it appears to reduce the risk of future episodes of depression.
Absence seizures are one of several kinds of generalized seizures. In the past, absence epilepsy was referred to as "pyknolepsy," a term derived from the Greek word "pyknos," signifying "extremely frequent" or "grouped". These seizures are sometimes referred to as petit mal seizures ; however, usage of this terminology is no longer recommended. Absence seizures are characterized by a brief loss and return of consciousness, generally not followed by a period of lethargy. Absence seizures are most common in children. They affect both sides of the brain.

Oxcarbazepine, sold under the brand name Trileptal among others, is a medication used to treat epilepsy. For epilepsy it is used for both focal seizures and generalized seizures. It has been used both alone and as add-on therapy in people with bipolar disorder who have had no success with other treatments. It is taken by mouth.
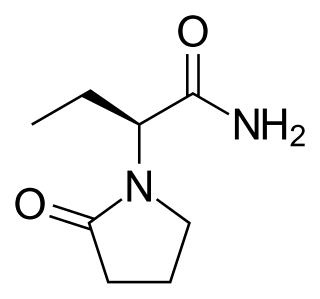
Levetiracetam, sold under the brand name Keppra among others, is a medication used to treat epilepsy. It is used for partial-onset, myoclonic, or tonic–clonic seizures and is taken either by mouth as an immediate or extended release formulation or by injection into a vein.
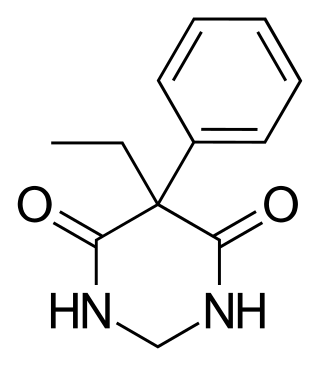
Primidone, sold under various brand names, is a barbiturate medication that is used to treat partial and generalized seizures and essential tremors. It is taken by mouth.
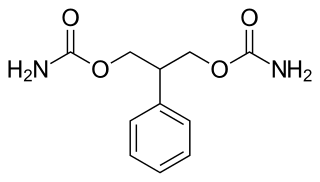
Felbamate is an anticonvulsant used in the treatment of epilepsy. It is used to treat partial seizures in adults and partial and generalized seizures associated with Lennox–Gastaut syndrome in children. However, an increased risk of potentially fatal aplastic anemia and/or liver failure limit the drug's usage to severe refractory epilepsy.

Generalized epilepsy is a form of epilepsy characterised by generalised seizures with no apparent cause. Generalized seizures, as opposed to focal seizures, are a type of seizure that impairs consciousness and distorts the electrical activity of the whole or a larger portion of the brain.
Progressive Myoclonic Epilepsies (PME) are a rare group of inherited neurodegenerative diseases characterized by myoclonus, resistance to treatment, and neurological deterioration. The cause of PME depends largely on the type of PME. Most PMEs are caused by autosomal dominant or recessive and mitochondrial mutations. The location of the mutation also affects the inheritance and treatment of PME. Diagnosing PME is difficult due to their genetic heterogeneity and the lack of a genetic mutation identified in some patients. The prognosis depends largely on the worsening symptoms and failure to respond to treatment. There is no current cure for PME and treatment focuses on managing myoclonus and seizures through antiepileptic medication (AED).

Lacosamide, sold under the brand name Vimpat among others, is a medication used for the treatment of partial-onset seizures and primary generalized tonic-clonic seizures. It is used by mouth or intravenously.

Eslicarbazepine acetate (ESL), sold under the brand names Aptiom and Zebinix among others, is an anticonvulsant medication approved for use in Europe and the United States as monotherapy or as additional therapy for partial-onset seizures epilepsy.

Phentermine/topiramate, sold under the brand name Qsymia, is a combination drug of phentermine and topiramate used to treat obesity. It is used together with dietary changes and exercise. If less than 3% weight loss is seen after 3 months it is recommended the medication be stopped. The weight loss is modest. Effects on heart related health problems or death is unclear.

Epilepsy is a neurological condition of recurrent episodes of unprovoked epileptic seizures. A seizure is an abnormal neuronal brain activity that can cause intellectual, emotional, and social consequences. Epilepsy affects children and adults of all ages and races, and is one of the most common neurological disorders of the nervous system. Epilepsy is more common among children than adults, affecting about 6 out of 1000 US children that are between the age of 0 to 5 years old. The epileptic seizures can be of different types depending on the part of the brain that was affected, seizures are classified in 2 main types partial seizure or generalized seizure.

Drospirenone/ethinylestradiol/levomefolic acid (EE/DRSP/LMF), sold under the brand name Beyaz among others, is a combination of ethinylestradiol (EE), an estrogen, drospirenone (DRSP), a progestogen, antimineralocorticoid, and antiandrogen, and levomefolic acid (LMF), a form of vitamin B9, which is used as a birth control pill to prevent pregnancy in women. The formulation contains folate as the calcium salt of levomefolic acid to lower the risk of complications such as fetal neural tube defects should the medication fail as a form of birth control. EE/DRSP/LMF was approved for use by the US Food and Drug Administration (FDA) in September 2010.
Jeavons syndrome is a type of epilepsy. It is one of the most distinctive reflex syndromes of idiopathic generalized epilepsy characterized by the triad of eyelid myoclonia with and without absences, eye-closure-induced seizures, EEG paroxysms, or both, and photosensitivity. Eyelid myoclonia with or without absences is a form of epileptic seizure manifesting with myoclonic jerks of the eyelids with or without a brief absence. These are mainly precipitated by closing of the eyes and lights. Eyelid myoclonia is the defining seizure type of Jeavons syndrome.
Combined hormonal contraception (CHC), or combined birth control, is a form of hormonal contraception which combines both an estrogen and a progestogen in varying formulations.













