
A brain tumor occurs when abnormal cells form within the brain. There are two main types of tumors: malignant tumors and benign (non-cancerous) tumors. These can be further classified as primary tumors, which start within the brain, and secondary tumors, which most commonly have spread from tumors located outside the brain, known as brain metastasis tumors. All types of brain tumors may produce symptoms that vary depending on the size of the tumor and the part of the brain that is involved. Where symptoms exist, they may include headaches, seizures, problems with vision, vomiting and mental changes. Other symptoms may include difficulty walking, speaking, with sensations, or unconsciousness.

An osteosarcoma (OS) or osteogenic sarcoma (OGS) is a cancerous tumor in a bone. Specifically, it is an aggressive malignant neoplasm that arises from primitive transformed cells of mesenchymal origin and that exhibits osteoblastic differentiation and produces malignant osteoid.

A thymoma is a tumor originating from the epithelial cells of the thymus that is considered a rare malignancy. Thymomas are frequently associated with neuromuscular disorders such as myasthenia gravis; thymoma is found in 20% of patients with myasthenia gravis. Once diagnosed, thymomas may be removed surgically. In the rare case of a malignant tumor, chemotherapy may be used.
Spinal tumors are neoplasms located in either the vertebral column or the spinal cord. There are three main types of spinal tumors classified based on their location: extradural and intradural. Extradural tumors are located outside the dura mater lining and are most commonly metastatic. Intradural tumors are located inside the dura mater lining and are further subdivided into intramedullary and extramedullary tumors. Intradural-intramedullary tumors are located within the dura and spinal cord parenchyma, while intradural-extramedullary tumors are located within the dura but outside the spinal cord parenchyma. The most common presenting symptom of spinal tumors is nocturnal back pain. Other common symptoms include muscle weakness, sensory loss, and difficulty walking. Loss of bowel and bladder control may occur during the later stages of the disease.

An ependymoma is a tumor that arises from the ependyma, a tissue of the central nervous system. Usually, in pediatric cases the location is intracranial, while in adults it is spinal. The common location of intracranial ependymomas is the fourth ventricle. Rarely, ependymomas can occur in the pelvic cavity.

Chondrosarcoma is a bone sarcoma, a primary cancer composed of cells derived from transformed cells that produce cartilage. A chondrosarcoma is a member of a category of tumors of bone and soft tissue known as sarcomas. About 30% of bone sarcomas are chondrosarcomas. It is resistant to chemotherapy and radiotherapy. Unlike other primary bone sarcomas that mainly affect children and adolescents, a chondrosarcoma can present at any age. It more often affects the axial skeleton than the appendicular skeleton.

Pseudomyxoma peritonei (PMP) is a clinical condition caused by cancerous cells that produce abundant mucin or gelatinous ascites. The tumors cause fibrosis of tissues and impede digestion or organ function, and if left untreated, the tumors and mucin they produce will fill the abdominal cavity. This will result in compression of organs and will destroy the function of the colon, small intestine, stomach, or other organs. Prognosis with treatment in many cases is optimistic, but the disease is lethal if untreated, with death occurring via cachexia, bowel obstruction, or other types of complications.

Desmoplastic small-round-cell tumor (DSRCT) is an aggressive and rare cancer that primarily occurs as masses in the abdomen. Other areas affected may include the lymph nodes, the lining of the abdomen, diaphragm, spleen, liver, chest wall, skull, spinal cord, large intestine, small intestine, bladder, brain, lungs, testicles, ovaries, and the pelvis. Reported sites of metastatic spread include the liver, lungs, lymph nodes, brain, skull, and bones. It is characterized by the EWS-WT1 fusion protein.

A hemangiopericytoma is a type of soft-tissue sarcoma that originates in the pericytes in the walls of capillaries. When inside the nervous system, although not strictly a meningioma tumor, it is a meningeal tumor with a special aggressive behavior. It was first characterized in 1942.
Adjuvant therapy, also known as adjunct therapy, adjuvant care, or augmentation therapy, is a therapy that is given in addition to the primary or initial therapy to maximize its effectiveness. The surgeries and complex treatment regimens used in cancer therapy have led the term to be used mainly to describe adjuvant cancer treatments. An example of such adjuvant therapy is the additional treatment usually given after surgery where all detectable disease has been removed, but where there remains a statistical risk of relapse due to the presence of undetected disease. If known disease is left behind following surgery, then further treatment is not technically adjuvant.
A blastoma is a type of cancer, more common in children, that is caused by malignancies in precursor cells, often called blasts. Examples are nephroblastoma, medulloblastoma, and retinoblastoma. The suffix -blastoma is used to imply a tumor of primitive, incompletely differentiated cells, e.g., chondroblastoma is composed of cells resembling the precursor of chondrocytes.

Mucoepidermoid carcinoma (MEC) is the most common type of minor salivary gland malignancy in adults. Mucoepidermoid carcinoma can also be found in other organs, such as bronchi, lacrimal sac, and thyroid gland.
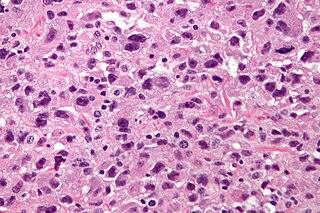
Undifferentiated pleomorphic sarcoma (UPS), also termed pleomorphic myofibrosarcoma, high-grade myofibroblastic sarcoma, and high-grade myofibrosarcoma, is characterized by the World Health Organization (WHO), 2020, as a rare, poorly differentiated neoplasm, i.e. an abnormal growth of cells that have an unclear identity and/or cell of origin. WHO classified it as one of the undifferentiated/unclassified sarcomas in the category of tumors of uncertain differentiation. Sarcomas are cancers known or thought to derive from mesenchymal stem cells that typically develop in bone, muscle, fat, blood vessels, lymphatic vessels, tendons, and ligaments. More than 70 sarcoma subtypes have been described. The UPS subtype of these sarcomas consists of tumor cells that are poorly differentiated and may appear as spindle-shaped cells, histiocytes, and giant cells. UPS is considered a diagnosis that defies formal sub-classification after thorough histologic, immunohistochemical, and ultrastructural examinations fail to identify the type of cells involved.

Thymic carcinoma, or type C thymoma, is a malignancy of the thymus. It is a rare cancer that is often diagnosed at advanced stages. Recurrence following treatment is common, and thymic carcinoma is associated with a poor prognosis.

An inverted papilloma, also known as Ringertz tumour, is a type of tumor in which surface epithelial cells grow downward into the underlying supportive tissue. It may occur in the nose and/or sinuses or in the urinary tract. When it occurs in the nose or sinuses, it may cause symptoms similar to those caused by sinusitis, such as nasal congestion. When it occurs in the urinary tract, it may cause blood in the urine.
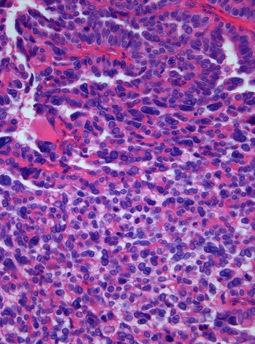
A choroid plexus carcinoma is a type of choroid plexus tumor that affects the choroid plexus of the brain. It is considered the worst of the three grades of chord plexus tumors, having a much poorer prognosis than choroid atypical plexus papilloma and choroid plexus papilloma. The disease creates lesions in the brain and increases cerebrospinal fluid volume, resulting in hydrocephalus.
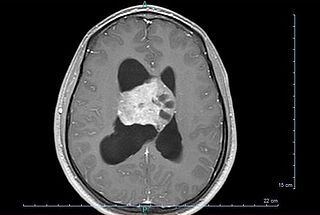
Central neurocytoma (CNC) is an extremely rare, ordinarily benign intraventricular brain tumour that typically forms from the neuronal cells of the septum pellucidum. The majority of central neurocytomas grow inwards into the ventricular system forming interventricular neurocytomas. This leads to two primary symptoms of CNCs, blurred vision and increased intracranial pressure. Treatment for a central neurocytoma typically involves surgical removal, with an approximate 1 in 5 chance of recurrence. Central neurocytomas are classified as a grade II tumor under the World Health Organization's classification of tumors of the nervous system.
Paranasal sinus and nasal cavity cancer is a type of cancer that is caused by the appearance and spread of malignant cells into the paranasal sinus and nasal cavity. The cancer most commonly occurs in people between 50 and 70 years old, and occurs twice as often in males as in females. During early phases of the cancer, symptoms may include nasal obstruction and hyposmia, as well as other symptoms. More symptoms may develop as malignant cells further grow and spread into other nearby tissue such as the palate or orbital floor. X-rays of the head and MRI can aid in diagnosis of the cancer while tumor resection surgery, radiation therapy and chemotherapy can be used for treatment of the cancer.
Myxofibrosarcoma (MFS), although a rare type of tumor, is one of the most common soft tissue sarcomas, i.e. cancerous tumors, that develop in the soft tissues of elderly individuals. Initially considered to be a type of histiocytoma termed fibrous histiocytoma or myxoid variant of malignant fibrous histiocytoma, Angervall et al. termed this tumor myxofibrosarcoma in 1977. In 2020, the World Health Organization reclassified MFS as a separate and distinct tumor in the category of malignant fibroblastic and myofibroblastic tumors.
Sclerosing epithelioid fibrosarcoma (SEF) is a very rare malignant tumor of soft tissues that on microscopic examination consists of small round or ovoid neoplastic epithelioid fibroblast-like cells, i.e. cells that have features resembling both epithelioid cells and fibroblasts. In 2020, the World Health Organization classified SEF as a distinct tumor type in the category of malignant fibroblastic and myofibroblastic tumors. However, current studies have reported that low-grade fibromyxoid sarcoma (LGFMS) has many clinically and pathologically important features characteristic of SEF; these studies suggest that LGSFMS may be an early form of, and over time progress to become, a SEF. Since the World Health Organization has classified LGFMS as one of the malignant fibroblastic and myofibroblastic tumors that is distinctly different than SEF, SEF and LGFMS are here regarded as different tumor forms.















