
Flow cytometry (FC) is a technique used to detect and measure physical and chemical characteristics of a population of cells or particles.

Hybridoma technology is a method for producing large numbers of identical antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal B cell cancer cells, a myeloma, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma. The hybridomas can be grown in culture, each culture starting with one viable hybridoma cell, producing cultures each of which consists of genetically identical hybridomas which produce one antibody per culture (monoclonal) rather than mixtures of different antibodies (polyclonal). The myeloma cell line that is used in this process is selected for its ability to grow in tissue culture and for an absence of antibody synthesis. In contrast to polyclonal antibodies, which are mixtures of many different antibody molecules, the monoclonal antibodies produced by each hybridoma line are all chemically identical.

Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33,342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similar excitation–emission spectra.
Immunophenotyping is a technique used to study the protein expressed by cells. This technique is commonly used in basic science research and laboratory diagnostic purpose. This can be done on tissue section, cell suspension, etc. An example is the detection of tumor markers, such as in the diagnosis of leukemia. It involves the labelling of white blood cells with antibodies directed against surface proteins on their membrane. By choosing appropriate antibodies, the differentiation of leukemic cells can be accurately determined. The labelled cells are processed in a flow cytometer, a laser-based instrument capable of analyzing thousands of cells per second. The whole procedure can be performed on cells from the blood, bone marrow or spinal fluid in a matter of a few hours.
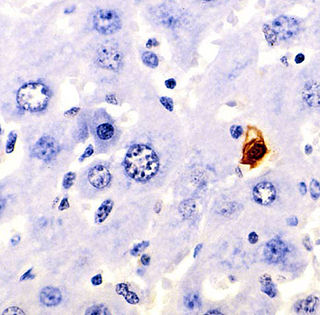
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) is a method for detecting DNA fragmentation by labeling the 3′- hydroxyl termini in the double-strand DNA breaks generated during apoptosis.
Phycobiliproteins are water-soluble proteins present in cyanobacteria and certain algae. They capture light energy, which is then passed on to chlorophylls during photosynthesis. Phycobiliproteins are formed of a complex between proteins and covalently bound phycobilins that act as chromophores. They are most important constituents of the phycobilisomes.

Annexin A5 is a cellular protein in the annexin group. In flow cytometry, annexin V is commonly used to detect apoptotic cells by its ability to bind to phosphatidylserine, a marker of apoptosis when it is on the outer leaflet of the plasma membrane. The function of the protein is unknown; however, annexin A5 has been proposed to play a role in the inhibition of blood coagulation by competing for phosphatidylserine binding sites with prothrombin and also to inhibit the activity of phospholipase A1. These properties have been found by in vitro experiments.

B-cell prolymphocytic leukemia, referred to as B-PLL, is a rare blood cancer. It is a more aggressive, but still treatable, form of leukemia.
Minimal residual disease (MRD) is the name given to small numbers of leukaemic cells that remain in the person during treatment, or after treatment when the patient is in remission. It is the major cause of relapse in cancer and leukemia. Up until a decade ago, none of the tests used to assess or detect cancer were sensitive enough to detect MRD. Now, however, very sensitive molecular biology tests are available, based on DNA, RNA or proteins. These can measure minute levels of cancer cells in tissue samples, sometimes as low as one cancer cell in a million normal cells.
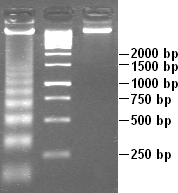
Apoptotic DNA fragmentation is a key feature of apoptosis, a type of programmed cell death. Apoptosis is characterized by the activation of endogenous endonucleases, particularly the caspase-3 activated DNase (CAD), with subsequent cleavage of nuclear DNA into internucleosomal fragments of roughly 180 base pairs (bp) and multiples thereof (360, 540 etc.). The apoptotic DNA fragmentation is being used as a marker of apoptosis and for identification of apoptotic cells either via the DNA laddering assay, the TUNEL assay, or the by detection of cells with fractional DNA content ("sub G1 cells") on DNA content frequency histograms e.g. as in the Nicoletti assay.

Anti-double stranded DNA (Anti-dsDNA) antibodies are a group of anti-nuclear antibodies (ANA) the target antigen of which is double stranded DNA. Blood tests such as enzyme-linked immunosorbent assay (ELISA) and immunofluorescence are routinely performed to detect anti-dsDNA antibodies in diagnostic laboratories. They are highly diagnostic of systemic lupus erythematosus (SLE) and are implicated in the pathogenesis of lupus nephritis.
The Streptamer technology allows the reversible isolation and staining of antigen-specific T cells. This technology combines a current T cell isolation method with the Strep-tag technology. In principle, the T cells are separated by establishing a specific interaction between the T cell of interest and a molecule that is conjugated to a marker, which enables the isolation. The reversibility of this interaction and the low temperatures at which it is performed allows for the isolation and characterization of functional T cells. Because T cells remain phenotypically and functionally indistinguishable from untreated cells, this method offers modern strategies in clinical and basic T cell research.

Cytometry is the measurement of number and characteristics of cells. Variables that can be measured by cytometric methods include cell size, cell count, cell morphology, cell cycle phase, DNA content, and the existence or absence of specific proteins on the cell surface or in the cytoplasm. Cytometry is used to characterize and count blood cells in common blood tests such as the complete blood count. In a similar fashion, cytometry is also used in cell biology research and in medical diagnostics to characterize cells in a wide range of applications associated with diseases such as cancer and AIDS.
Cell cycle analysis by DNA content measurement is a method that most frequently employs flow cytometry to distinguish cells in different phases of the cell cycle. Before analysis, the cells are usually permeabilised and treated with a fluorescent dye that stains DNA quantitatively, such as propidium iodide (PI) or 4,6-diamidino-2-phenylindole (DAPI). The fluorescence intensity of the stained cells correlates with the amount of DNA they contain. As the DNA content doubles during the S phase, the DNA content (and thereby intensity of fluorescence) of cells in the G0 phase and G1 phase (before S), in the S phase, and in the G2 phase and M phase (after S) identifies the cell cycle phase position in the major phases (G0/G1 versus S versus G2/M phase) of the cell cycle. The cellular DNA content of individual cells is often plotted as their frequency histogram to provide information about relative frequency (percentage) of cells in the major phases of the cell cycle.

Proximity ligation assay is a technology that extends the capabilities of traditional immunoassays to include direct detection of proteins, protein interactions, Extracellular vesicles and post translational modifications with high specificity and sensitivity. Protein targets can be readily detected and localized with single molecule resolution and objectively quantified in unmodified cells and tissues. Utilizing only a few cells, sub-cellular events, even transient or weak interactions, are revealed in situ and sub-populations of cells can be differentiated. Within hours, results from conventional co-immunoprecipitation and co-localization techniques can be confirmed.
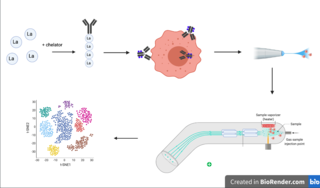
Cytometry by time of flight, or CyTOF, is an application of mass cytometry used to quantify labeled targets on the surface and interior of single cells. CyTOF allows the quantification of multiple cellular components simultaneously using an ICP-MS detector.
MHC multimers are oligomeric forms of MHC molecules, designed to identify and isolate T-cells with high affinity to specific antigens amid a large group of unrelated T-cells. Multimers generally range in size from dimers to octamers; however, some companies use even higher quantities of MHC per multimer. Multimers may be used to display class 1 MHC, class 2 MHC, or nonclassical molecules from species such as monkeys, mice, and humans.
Flow cytometry bioinformatics is the application of bioinformatics to flow cytometry data, which involves storing, retrieving, organizing and analyzing flow cytometry data using extensive computational resources and tools. Flow cytometry bioinformatics requires extensive use of and contributes to the development of techniques from computational statistics and machine learning. Flow cytometry and related methods allow the quantification of multiple independent biomarkers on large numbers of single cells. The rapid growth in the multidimensionality and throughput of flow cytometry data, particularly in the 2000s, has led to the creation of a variety of computational analysis methods, data standards, and public databases for the sharing of results.
Complement-dependent cytotoxicity (CDC) is an effector function of IgG and IgM antibodies. When they are bound to surface antigen on target cell, the classical complement pathway is triggered by bonding protein C1q to these antibodies, resulting in formation of a membrane attack complex (MAC) and target cell lysis.
Pig-a gene mutation assay is a flow cytometry-based method for detecting mammalian cells that have inactivating mutations in the endogenous X-linked reporter gene called phosphatidyl inositolglycan class A gene. PIG-A is involved in the synthesis of glycosylphosphatidylinositol (GPI), an anchor molecule that tethers multiple protein marker molecules at the surface of the cells. When the sample containing wild-type and PIG-A mutant cells is labeled with fluorescent antibodies raised against GPI-anchored protein markers the wild-type cells will fluoresce and PIG-A mutant cells will not. The fraction of non-fluorescent PIG-A mutant cells in the antibody-labeled sample can be efficiently determined on any of the modern high throughput flow cytometers. The PIG-A mutant frequency can be determined with high accuracy within minutes by processing samples containing a total of a million cells or more.











