Related Research Articles
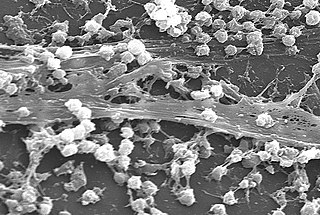
A biofilm is an syntrophic community of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric combination of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".
In biology, quorum sensing or quorum signaling (QS) is the ability to detect and respond to cell population density by gene regulation. Quorum sensing is a type of cellular signaling, and more specifically can be considered a type of paracrine signaling. However, it also contains traits of both autocrine signaling: a cell produces both the autoinducer molecule and the receptor for the autoinducer. As one example, QS enables bacteria to restrict the expression of specific genes to the high cell densities at which the resulting phenotypes will be most beneficial, especially for phenotypes that would be ineffective at low cell densities and therefore too energetically costly to express. Many species of bacteria use quorum sensing to coordinate gene expression according to the density of their local population. In a similar fashion, some social insects use quorum sensing to determine where to nest. Quorum sensing in pathogenic bacteria activates host immune signaling and prolongs host survival, by limiting the bacterial intake of nutrients, such as tryptophan, which further is converted to serotonin. As such, quorum sensing allows a commensal interaction between host and pathogenic bacteria. Quorum sensing may also be useful for cancer cell communications.

Aliivibrio fischeri is a Gram-negative, rod-shaped bacterium found globally in marine environments. This species has bioluminescent properties, and is found predominantly in symbiosis with various marine animals, such as the Hawaiian bobtail squid. It is heterotrophic, oxidase-positive, and motile by means of a single polar flagella. Free-living A. fischeri cells survive on decaying organic matter. The bacterium is a key research organism for examination of microbial bioluminescence, quorum sensing, and bacterial-animal symbiosis. It is named after Bernhard Fischer, a German microbiologist.

Vibrio harveyi is a Gram-negative, bioluminescent, marine bacterium in the genus Vibrio. V. harveyi is rod-shaped, motile, facultatively anaerobic, halophilic, and competent for both fermentative and respiratory metabolism. It does not grow below 4 °C. V. harveyi can be found free-swimming in tropical marine waters, commensally in the gut microflora of marine animals, and as both a primary and opportunistic pathogen of marine animals, including Gorgonian corals, oysters, prawns, lobsters, the common snook, barramundi, turbot, milkfish, and seahorses. It is responsible for luminous vibriosis, a disease that affects commercially farmed penaeid prawns. Additionally, based on samples taken by ocean-going ships, V. harveyi is thought to be the cause of the milky seas effect, in which, during the night, a uniform blue glow is emitted from the seawater. Some glows can cover nearly 6,000 sq mi (16,000 km2).

N-Acyl homoserine lactones are a class of signaling molecules involved in bacterial quorum sensing, a means of communication between bacteria enabling behaviors based on population density.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes. P. aeruginosa is able to selectively inhibit various antibiotics from penetrating its outer membrane - and has high resistance to several antibiotics, according to the World Health Organization P. aeruginosa poses one of the greatest threats to humans in terms of antibiotic resistance.
Autoinducers are signaling molecules that are produced in response to changes in cell-population density. As the density of quorum sensing bacterial cells increases so does the concentration of the autoinducer. Detection of signal molecules by bacteria acts as stimulation which leads to altered gene expression once the minimal threshold is reached. Quorum sensing is a phenomenon that allows both Gram-negative and Gram-positive bacteria to sense one another and to regulate a wide variety of physiological activities. Such activities include symbiosis, virulence, motility, antibiotic production, and biofilm formation. Autoinducers come in a number of different forms depending on the species, but the effect that they have is similar in many cases. Autoinducers allow bacteria to communicate both within and between different species. This communication alters gene expression and allows bacteria to mount coordinated responses to their environments, in a manner that is comparable to behavior and signaling in higher organisms. Not surprisingly, it has been suggested that quorum sensing may have been an important evolutionary milestone that ultimately gave rise to multicellular life forms.

Lactonase (EC 3.1.1.81, acyl-homoserine lactonase; systematic name N-acyl-L-homoserine-lactone lactonohydrolase) is a metalloenzyme, produced by certain species of bacteria, which targets and inactivates acylated homoserine lactones (AHLs). It catalyzes the reaction

Autoinducer-2 (AI-2), a furanosyl borate diester or tetrahydroxy furan, is a member of a family of signaling molecules used in quorum sensing. AI-2 is one of only a few known biomolecules incorporating boron. First identified in the marine bacterium Vibrio harveyi, AI-2 is produced and recognized by many Gram-negative and Gram-positive bacteria. AI-2 arises by the reaction of 4,5-dihydroxy-2,3-pentanedione, which is produced enzymatically, with boric acid and is recognized by the two-component sensor kinase LuxPQ in Vibrionaceae.
Roberto Kolter is Professor of Microbiology, Emeritus at Harvard Medical School, an author, and past president of the American Society for Microbiology. Kolter has been a professor at Harvard Medical School since 1983 and was Co-director of Harvard's Microbial Sciences Initiative from 2003-2018. During the 35-year term of the Kolter laboratory from 1983 to 2018, more than 130 graduate student and postdoctoral trainees explored an eclectic mix of topics gravitating around the study of microbes. Kolter is a fellow of the American Association for the Advancement of Science and of the American Academy of Microbiology.

Rhamnolipids are a class of glycolipid produced by Pseudomonas aeruginosa, amongst other organisms, frequently cited as bacterial surfactants. They have a glycosyl head group, in this case a rhamnose moiety, and a 3-(hydroxyalkanoyloxy)alkanoic acid (HAA) fatty acid tail, such as 3-hydroxydecanoic acid.
Interspecies quorum sensing is a type of quorum sensing in which bacteria send and receive signals to other species besides their own. This is accomplished by the secretion of signaling molecules which trigger a response in nearby bacteria at high enough concentrations. Once the molecule hits a certain concentration it triggers the transcription of certain genes such as virulence factors. It has been discovered that bacteria can not only interact via quorum sensing with members of their own species but that there is a kind of universal molecule that allows them to gather information about other species as well. This universal molecule is called autoinducer 2 or AI-2.
Acyl-homoserine-lactone synthase is an enzyme with systematic name acyl-(acyl-carrier protein):S-adenosyl-L-methionine acyltranserase . This enzyme catalyses the following chemical reaction

Bioluminescent bacteria are light-producing bacteria that are predominantly present in sea water, marine sediments, the surface of decomposing fish and in the gut of marine animals. While not as common, bacterial bioluminescence is also found in terrestrial and freshwater bacteria. These bacteria may be free living or in symbiosis with animals such as the Hawaiian Bobtail squid or terrestrial nematodes. The host organisms provide these bacteria a safe home and sufficient nutrition. In exchange, the hosts use the light produced by the bacteria for camouflage, prey and/or mate attraction. Bioluminescent bacteria have evolved symbiotic relationships with other organisms in which both participants benefit close to equally. Another possible reason bacteria use luminescence reaction is for quorum sensing, an ability to regulate gene expression in response to bacterial cell density.
The type VI secretion system (T6SS) is molecular machine used by a wide range of Gram-negative bacterial species to transport effectors from the interior of a bacterial cell across the cellular envelope into an adjacent target cell. While often reported that the T6SS was discovered in 2006 by researchers studying the causative agent of cholera, Vibrio cholerae, the first study demonstrating that T6SS genes encode a protein export apparatus was actually published in 2004, in a study of protein secretion by the fish pathogen Edwardsiella tarda.
Barbara Hotham Iglewski was an American microbiologist. She was director of international programs at the University of Rochester Medical Center where she was a professor of microbiology and immunology.
Karine Gibbs is a Jamaican American microbiologist and immunologist and an associate professor in the Department of Plant and Microbial Biology at the University of California, Berkeley. Gibbs’ research merges the fields of sociomicrobiology and bacterial cell biology to explore how the bacterial pathogen Proteus mirabilis, a common gut bacterium which can become pathogenic and cause urinary tract infections, identifies self versus non-self. In 2013, Gibbs and her team were the first to sequence the genome of P. mirabilis BB2000, the model organism for studying self-recognition. In graduate school at Stanford University, Gibbs helped to pioneer the design of a novel tool that allowed for visualization of the movement of bacterial membrane proteins in real time. In 2020, Gibbs was recognized by Cell Press as one of the top 100 Inspiring Black Scientists in America.
Kalai Mathee is a professor at Florida International University, joint editor-in-chief of the Journal of Medical Microbiology, and an elected fellow of the American Academy of Microbiology. She is known for her research on bacterial infections caused by Pseudomonas aeruginosa.
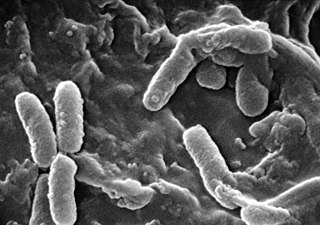
The molecule 2-heptyl-3-hydroxy-4-quinolone, also named the Pseudomonas quinolone signal (PQS), has been discovered as an intracellular link between the two major quorum sensing systems of P. aeruginosa; the las and rhl systems. These systems together control expression of virulence factors and play a major role in the formation of biofilms in Pseudomonas aeruginosa. P. aeruginosa is a gram-negative bacteria and opportunistic human pathogen that can cause serious and sometimes fatal infections in humans. Similar to other bacterial species, P. aeruginosa uses quorum sensing (QS) systems to communicate between cells in a population. This allows coordination of gene expression in a population based on changing cell densities, abundance of nutrients, and other environmental factors.
References
- 1 2 "Jeffrey I. Gordon, Peter Greenberg and Bonnie L. Bassler, Princess of Asturias Award for Technical and Scientific Research". Princess of Asturias Foundation. July 6, 2023. Archived from the original on August 8, 2023. Retrieved August 8, 2023.
- 1 2 3 4 5 6 Davis, Tinsley H. (2004). "Biography of E. P. Greenberg". Proceedings of the National Academy of Sciences . 101 (45): 15830–15832. doi: 10.1073/pnas.0407738101 . PMC 528758 . PMID 15520366.
- 1 2 3 "Everett Peter Greenberg". Frontiers Media. Archived from the original on August 8, 2023. Retrieved August 8, 2023.
- 1 2 3 4 5 6 7 "Autobiography of E Peter Greenberg". Shaw Prize. Archived from the original on August 9, 2023. Retrieved August 9, 2023.
- ↑ "Eavesdropping on microbe chatter earns Gairdner Award". University of Washington School of Medicine. March 29, 2023. Archived from the original on August 9, 2023. Retrieved August 9, 2023.
- ↑ "Distinguished molecular microbiologist to chair department at UW School of Medicine". University of Washington. October 8, 2004. Archived from the original on August 21, 2023. Retrieved August 21, 2023.
- ↑ Ornston, Nick (2001). "Preface by Nick Ornston". Annual Review of Microbiology . 55. doi: 10.1146/annurev.mi.55.010101.100001 .
- ↑ Greenberg, E. Peter (2003). "Bacterial communication: Tiny teamwork". Nature . 424 (6945): 134. Bibcode:2003Natur.424..134G. doi: 10.1038/424134a . PMID 12853935.
- ↑ Bassler, Bonnie L.; Losick, Richard (2006). "Bacterially Speaking". Cell . 125 (2): 237–246. doi: 10.1016/j.cell.2006.04.001 . PMID 16630813.
- ↑ Tomasz, Alexander (1965). "Control of the competent state in Pneumococcus by a hormone-like cell product: an example for a new type of regulatory mechanism in bacteria". Nature. 208 (5006): 155–159. Bibcode:1965Natur.208..155T. doi:10.1038/208155a0. PMID 5884251. S2CID 4202362 . Retrieved August 11, 2023.
- ↑ Nealson, Kenneth H.; Platt, Terry; Hastings, J. Woodland (1970). "Cellular Control of the Synthesis and Activity of the Bacterial Luminescent System". Journal of Bacteriology . 104 (1): 313–322. doi: 10.1128/jb.104.1.313-322.1970 . PMC 248216 . PMID 5473898.
- ↑ Kaplan, Heidi B.; Greenberg, E. P. (1985). "Diffusion of Autoinducer Is Involved in Regulation of the Vibrio fischeri Luminescence System". Journal of Bacteriology. 163 (3): 1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985 . PMC 219261 . PMID 3897188.
- 1 2 Whiteley, Marvin; Diggle, Stephen P.; Greenberg, E. Peter (2017). "Progress in and promise of bacterial quorum sensing research". Nature. 551 (7680): 313–320. Bibcode:2017Natur.551..313W. doi:10.1038/nature24624. PMC 5870893 . PMID 29144467.
- ↑ Choi, S. H.; Greenberg, E. P. (1991). "The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain". Proceedings of the National Academy of Sciences. 88 (24): 11115–11119. Bibcode:1991PNAS...8811115C. doi: 10.1073/pnas.88.24.11115 . PMC 53084 . PMID 1763027.
- ↑ Hanzelka, Brian L.; Greenberg, E. P. (1995). "Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain". Journal of Bacteriology. 177 (3): 815–817. doi: 10.1128/jb.177.3.815-817.1995 . PMC 176662 . PMID 7836318.
- ↑ Fuqua, W. Claiborne; Winans, Stephen C.; Greenberg, E. Peter (1994). "Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators". Journal of Bacteriology. 176 (2): 269–275. doi: 10.1128/jb.176.2.269-275.1994 . PMC 205046 . PMID 8288518.
- ↑ Gambello, Michael J.; Iglewski, Barbara H. (1991). "Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression". Journal of Bacteriology. 173 (9): 3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991 . PMC 207884 . PMID 1902216.
- ↑ Miranda, Samantha Wellington; Asfahl, Kyle L.; Dandekar, Ajai A.; Greenberg, E. P. (2022). "Pseudomonas aeruginosa Quorum Sensing". In Filloux, Alain; Ramos, Juan-Luis (eds.). Pseudomonas aeruginosa: Biology, Pathogenesis and Control Strategies. Advances in Experimental Medicine and Biology. Vol. 1386. Springer Science+Business Media. pp. 95–115. doi:10.1007/978-3-031-08491-1_4. ISBN 9783031084904. PMC 9942581 . PMID 36258070.
- ↑ Davies, David G.; Parsek, Matthew R.; Pearson, James P.; Iglewski, Barbara H.; Costerton, J. W.; Greenberg, E. P. (1998). "The Involvement of Cell-to-Cell Signals in the Development of a Bacterial Biofilm". Science . 280 (5361): 295–298. Bibcode:1998Sci...280..295D. doi:10.1126/science.280.5361.295. PMID 9535661 . Retrieved August 21, 2023.
- ↑ Singh, Pradeep K.; Schaefer, Amy L.; Parsek, Matthew R.; Moninger, Thomas O.; Welsh, Michael J.; Greenberg, E. P. (2000). "Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms". Nature. 407 (6805): 762–764. Bibcode:2000Natur.407..762S. doi:10.1038/35037627. PMID 11048725. S2CID 4372096 . Retrieved August 21, 2023.
- ↑ Banin, Ehud; Vasil, Michael L.; Greenberg, E. Peter (2005). "Iron and Pseudomonas aeruginosa biofilm formation". Proceedings of the National Academy of Sciences. 102 (31): 11076–11081. Bibcode:2005PNAS..10211076B. doi: 10.1073/pnas.0504266102 . PMC 1182440 . PMID 16043697.
- ↑ "Caroline (Carrie) Harwood". Archived from the original on August 10, 2023. Retrieved August 10, 2023.
- ↑ "Elected Fellows". American Association for the Advancement of Science. Archived from the original on August 18, 2023. Retrieved August 18, 2023.
- ↑ "Everett Peter Greenberg". American Academy of Arts and Sciences. Archived from the original on August 18, 2023. Retrieved August 18, 2023.
- ↑ "E. Peter Greenberg". National Academy of Sciences. Archived from the original on August 18, 2023. Retrieved August 18, 2023.
- ↑ "The 2015 Prize in Life Science & Medicine". Shaw Prize. Archived from the original on August 18, 2023. Retrieved August 18, 2023.
- ↑ "E Peter Greenberg". Gairdner Foundation. Archived from the original on August 11, 2023. Retrieved August 11, 2023.
