
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.
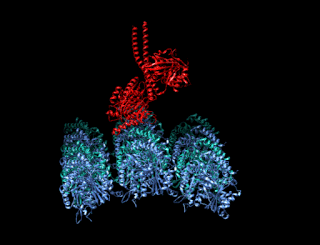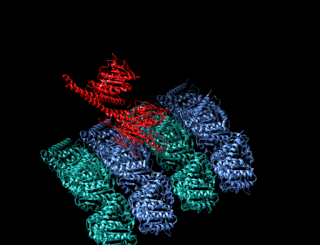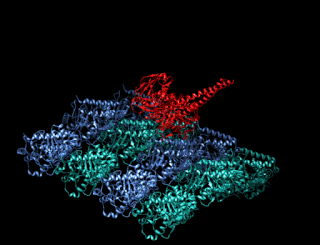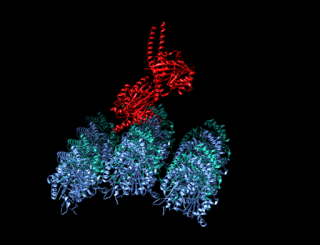
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells. Kinesins move along microtubule (MT) filaments and are powered by the hydrolysis of adenosine triphosphate (ATP). The active movement of kinesins supports several cellular functions including mitosis, meiosis and transport of cellular cargo, such as in axonal transport, and intraflagellar transport. Most kinesins walk towards the plus end of a microtubule, which, in most cells, entails transporting cargo such as protein and membrane components from the center of the cell towards the periphery. This form of transport is known as anterograde transport. In contrast, dyneins are motor proteins that move toward the minus end of a microtubule in retrograde transport.

Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport.

Molecular motors are natural (biological) or artificial molecular machines that are the essential agents of movement in living organisms. In general terms, a motor is a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors harness the chemical free energy released by the hydrolysis of ATP in order to perform mechanical work. In terms of energetic efficiency, this type of motor can be superior to currently available man-made motors. One important difference between molecular motors and macroscopic motors is that molecular motors operate in the thermal bath, an environment in which the fluctuations due to thermal noise are significant.

Axonal transport, also called axoplasmic transport or axoplasmic flow, is a cellular process responsible for movement of mitochondria, lipids, synaptic vesicles, proteins, and other organelles to and from a neuron's cell body, through the cytoplasm of its axon called the axoplasm. Since some axons are on the order of meters long, neurons cannot rely on diffusion to carry products of the nucleus and organelles to the end of their axons. Axonal transport is also responsible for moving molecules destined for degradation from the axon back to the cell body, where they are broken down by lysosomes.

Motor proteins are a class of molecular motors that can move along the cytoplasm of cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.
Plus-end-directed kinesin ATPase (EC 3.6.4.4, kinesin) is an enzyme with systematic name kinesin ATP phosphohydrolase (plus-end-directed). This enzyme catalyses the following chemical reaction

Dynactin is a 23 subunit protein complex that acts as a co-factor for the microtubule motor cytoplasmic dynein-1. It is built around a short filament of actin related protein-1 (Arp1).

Kinesin family member 5B (KIF5B) is a protein that in humans is encoded by the KIF5B gene. It is part of the kinesin family of motor proteins.

Kinesin-like protein KIF3B is a protein that in humans is encoded by the KIF3B gene. KIF3B is an N-type protein that complexes with two other kinesin proteins to form two-headed anterograde motors. First, KIF3B forms a heterodimer with KIF3A ; (KIF3A/3B), that is membrane-bound and has ATPase activity. Then KIFAP3 binds to the tail domain to form a heterotrimeric motor. This motor has a plus end-directed microtubule sliding activity that exhibits a velocity of ~0.3 μm/s a. There are 14 kinesin protein families in the kinesin superfamily and KIF3B is part of the Kinesin-2 family, of kinesins that can all form heterotrimeric complexes. Expression of the three motor subunits is ubiquitous. The KIG3A/3B/KAP3 motors can transport 90 to 160 nm in diameter organelles.

Kinesin-like protein KIF11 is a molecular motor protein that is essential in mitosis. In humans it is coded for by the gene KIF11. Kinesin-like protein KIF11 is a member of the kinesin superfamily, which are nanomotors that move along microtubule tracks in the cell. Named from studies in the early days of discovery, it is also known as Kinesin-5, or as BimC, Eg5 or N-2, based on the founding members of this kinesin family.

Intracellular transport is the movement of vesicles and substances within a cell. Intracellular transport is required for maintaining homeostasis within the cell by responding to physiological signals. Proteins synthesized in the cytosol are distributed to their respective organelles, according to their specific amino acid’s sorting sequence. Eukaryotic cells transport packets of components to particular intracellular locations by attaching them to molecular motors that haul them along microtubules and actin filaments. Since intracellular transport heavily relies on microtubules for movement, the components of the cytoskeleton play a vital role in trafficking vesicles between organelles and the plasma membrane by providing mechanical support. Through this pathway, it is possible to facilitate the movement of essential molecules such as membrane‐bounded vesicles and organelles, mRNA, and chromosomes.

Anna Sergeevna Akhmanova is a Russian-born professor of Cell Biology at Utrecht University in the Netherlands. She is best known for her research regarding microtubules and the proteins, called TIPs, that stabilize one specific end of the tubules. Among the awards she has won, she was one of the recipients of the 2018 Spinoza Prize, the highest honor for Dutch scientists.

Ian Read Gibbons, was a biophysicist and cell biologist. He discovered and named dynein, and demonstrated energy source as ATP is sufficient for dynein to walk on microtubules. In 2017, he and Ronald Vale received the Shaw Prize for their research on microtubule motor proteins.

Andrew P. Carter is a British structural biologist who works at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) in Cambridge, UK. He is known for his work on the microtubule motor dynein.
Samara Reck-Peterson is an American cell biologist and biophysicist. She is a Professor of Cellular and Molecular Medicine and Cell and Developmental Biology at the University of California, San Diego and an Investigator of the Howard Hughes Medical Institute. She is known for her contributions to our understanding of how dynein, an exceptionally large motor protein that moves many intracellular cargos, works and is regulated. She developed one of the first systems to produce recombinant dynein and discovered that, unlike other cytoskeletal motors, dynein can take a wide variety of step sizes, forward and back and even sideways. She lives in San Diego, California.
Edwin W. Taylor is an adjunct professor of cell and developmental biology at Northwestern University. He was elected to the National Academy of Sciences in 2001. Taylor received a BA in physics and chemistry from the University of Toronto in 1952; an MSc in physical chemistry from McMaster University in 1955, and a PhD in biophysics from the University of Chicago in 1957. In 2001 Taylor was elected to the National Academy of Scineces in Cellular and Developmental Biology and Biochemistry.

Erika L F. Holzbaur is an American biologist who is the William Maul Measey Professor of Physiology at University of Pennsylvania Perelman School of Medicine. Her research considers the dynamics of organelle motility along cytoskeleton of cells. She is particularly interested in the molecular mechanisms that underpin neurodegenerative diseases.
J. Richard McIntosh is a Distinguished Professor Emeritus in Molecular, Cellular, and Developmental Biology at the University of Colorado Boulder. McIntosh first graduated from Harvard with a BA in Physics in 1961, and again with a Ph.D. in Biophysics in 1968. He began his teaching career at Harvard but has spent most of his career at the University of Colorado Boulder. At the University of Colorado Boulder, McIntosh taught biology courses at both the undergraduate and graduate levels. Additionally, he created an undergraduate course in the biology of cancer towards the last several years of his teaching career. McIntosh's research career looks at a variety of things, including different parts of mitosis, microtubules, and motor proteins.
















