Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events. Most commonly, protein phosphorylation is catalyzed by protein kinases, ultimately resulting in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.

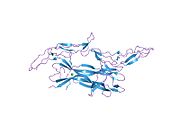
Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical nerve growth factor (NGF), a family which also includes NT-3 and NT-4/NT-5. Neurotrophic factors are found in the brain and the periphery. BDNF was first isolated from a pig brain in 1982 by Yves-Alain Barde and Hans Thoenen.

Neurotrophins are a family of proteins that induce the survival, development, and function of neurons.

Protein kinase B (PKB), also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription, and cell migration.

Tropomyosin receptor kinase A (TrkA), also known as high affinity nerve growth factor receptor, neurotrophic tyrosine kinase receptor type 1, or TRK1-transforming tyrosine kinase protein is a protein that in humans is encoded by the NTRK1 gene.

Tropomyosin receptor kinase B (TrkB), also known as tyrosine receptor kinase B, or BDNF/NT-3 growth factors receptor or neurotrophic tyrosine kinase, receptor, type 2 is a protein that in humans is encoded by the NTRK2 gene. TrkB is a receptor for brain-derived neurotrophic factor (BDNF). The standard pronunciation for this protein is "track bee".

The p75 neurotrophin receptor (p75NTR) was first identified in 1973 as the low-affinity nerve growth factor receptor (LNGFR) before discovery that p75NTR bound other neurotrophins equally well as nerve growth factor. p75NTR is a neurotrophic factor receptor. Neurotrophic factor receptors bind Neurotrophins including Nerve growth factor, Neurotrophin-3, Brain-derived neurotrophic factor, and Neurotrophin-4. All neurotrophins bind to p75NTR. This also includes the immature pro-neurotrophin forms. Neurotrophic factor receptors, including p75NTR, are responsible for ensuring a proper density to target ratio of developing neurons, refining broader maps in development into precise connections. p75NTR is involved in pathways that promote neuronal survival and neuronal death.
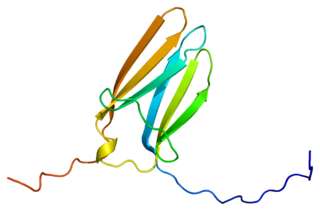
Tropomyosin receptor kinase C (TrkC), also known as NT-3 growth factor receptor, neurotrophic tyrosine kinase receptor type 3, or TrkC tyrosine kinase is a protein that in humans is encoded by the NTRK3 gene.
Neurotrophic factors (NTFs) are a family of biomolecules – nearly all of which are peptides or small proteins – that support the growth, survival, and differentiation of both developing and mature neurons. Most NTFs exert their trophic effects on neurons by signaling through tyrosine kinases, usually a receptor tyrosine kinase. In the mature nervous system, they promote neuronal survival, induce synaptic plasticity, and modulate the formation of long-term memories. Neurotrophic factors also promote the initial growth and development of neurons in the central nervous system and peripheral nervous system, and they are capable of regrowing damaged neurons in test tubes and animal models. Some neurotrophic factors are also released by the target tissue in order to guide the growth of developing axons. Most neurotrophic factors belong to one of three families: (1) neurotrophins, (2) glial cell-line derived neurotrophic factor family ligands (GFLs), and (3) neuropoietic cytokines. Each family has its own distinct cell signaling mechanisms, although the cellular responses elicited often do overlap.

Neurotrophin-3 is a protein that in humans is encoded by the NTF3 gene.
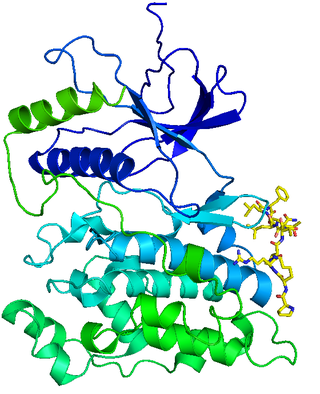
c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. They belong to the mitogen-activated protein kinase family, and are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock. They also play a role in T cell differentiation and the cellular apoptosis pathway. Activation occurs through a dual phosphorylation of threonine (Thr) and tyrosine (Tyr) residues within a Thr-Pro-Tyr motif located in kinase subdomain VIII. Activation is carried out by two MAP kinase kinases, MKK4 and MKK7, and JNK can be inactivated by Ser/Thr and Tyr protein phosphatases. It has been suggested that this signaling pathway contributes to inflammatory responses in mammals and insects.

Fibroblast growth factor receptor substrate 2 is a protein that in humans is encoded by the FRS2 gene.
Trk receptors are a family of tyrosine kinases that regulates synaptic strength and plasticity in the mammalian nervous system. Trk receptors affect neuronal survival and differentiation through several signaling cascades. However, the activation of these receptors also has significant effects on functional properties of neurons.

Cytoplasmic protein NCK2 is a protein that in humans is encoded by the NCK2 gene.
Neurotrophic factor receptors or neurotrophin receptors are a group of growth factor receptors which specifically bind to neurotrophins.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.
In cellular biology, dependence receptors are proteins that mediate programmed cell death by monitoring the absence of certain trophic factors that otherwise serve as ligands (interactors) for the dependence receptors. A trophic ligand is a molecule whose protein binding stimulates cell growth, differentiation, and/or survival. Cells depend for their survival on stimulation that is mediated by various receptors and sensors, and integrated via signaling within the cell and between cells. The withdrawal of such trophic support leads to a form of cellular suicide.

BNN-27, also known as 17α,20R-epoxypregn-5-ene-3β,21-diol, is a synthetic neurosteroid and "microneurotrophin" and analogue of the endogenous neurosteroid dehydroepiandrosterone (DHEA). It acts as a selective, high-affinity, centrally active agonist of the TrkA and p75NTR, receptors for nerve growth factor (NGF) and other neurotrophins, as well as for DHEA and DHEA sulfate (DHEA-S). BNN-27 has neuroprotective and neurogenic effects and has been suggested as a potential novel treatment for neurodegenerative diseases and brain trauma.

Daniel Djakiew is a scholar, researcher, teacher, and tenured full professor in the Department of Biochemistry and Molecular & Cellular Biology, School of Medicine, Georgetown University, Washington DC.
Neurotrophin mimetics are small molecules or peptide like molecules that can modulate the action of the neurotrophin receptor. One of the main causes of neurodegeneration involves changes in the expression of neurotrophins (NTs) and/or their receptors. Indeed, these imbalances or changes in their activity, lead to neuronal damage resulting in neurological and neurodegenerative conditions. The therapeutic properties of neurotrophins attracted the focus of many researchers during the years, but the poor pharmacokinetic properties, such as reduced bioavailability and low metabolic stability, the hyperalgesia, the inability to penetrate the blood–brain barrier and the short half-lives render the large neurotrophin proteins not suitable to be implemented as drugs.