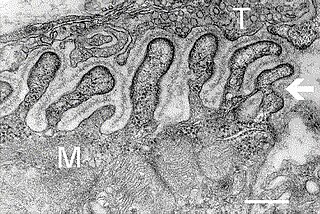
A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.
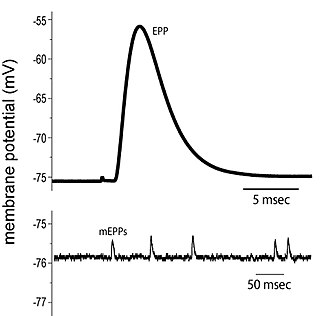
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.
MuSK is a receptor tyrosine kinase required for the formation and maintenance of the neuromuscular junction. It is activated by a nerve-derived proteoglycan called agrin, which is similarly also required for neuromuscular junction formation.
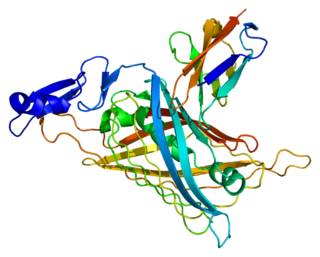
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. The name perlecan comes from its appearance as a "string of pearls" in rotary shadowed images.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. It is in this form that HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.
George D. Yancopoulos is a Greek-American biomedical scientist who is the co-founder, president and chief scientific officer of Regeneron Pharmaceuticals.
Dok-7 is a non-catalytic cytoplasmic adaptor protein that is expressed specifically in muscle and is essential for the formation of neuromuscular synapses. Further, Dok-7 contains pleckstrin homology (PH) and phosphotyrosine-binding (PTB) domains that are critical for Dok-7 function. Finally, mutations in Dok-7 are commonly found in patients with limb-girdle congenital myasthenia.

Dystroglycan is a protein that in humans is encoded by the DAG1 gene.

CTGF, also known as CCN2 or connective tissue growth factor, is a matricellular protein of the CCN family of extracellular matrix-associated heparin-binding proteins. CTGF has important roles in many biological processes, including cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair, and is critically involved in fibrotic disease and several forms of cancers.

Syndecan 1 is a protein which in humans is encoded by the SDC1 gene. The protein is a transmembrane heparan sulfate proteoglycan and is a member of the syndecan proteoglycan family. The syndecan-1 protein functions as an integral membrane protein and participates in cell proliferation, cell migration and cell-matrix interactions via its receptor for extracellular matrix proteins. Syndecan-1 is a sponge for growth factors and chemokines, with binding largely via heparan sulfate chains. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization and syndecan receptors are required for internalization of the HIV-1 tat protein.
Congenital myasthenic syndrome (CMS) is an inherited neuromuscular disorder caused by defects of several types at the neuromuscular junction. The effects of the disease are similar to Lambert-Eaton Syndrome and myasthenia gravis, the difference being that CMS is not an autoimmune disorder. There are only 600 known family cases of this disorder and it is estimated that its overall frequency in the human population is 1 in 200,000.

Syndecan-4 is a protein that in humans is encoded by the SDC4 gene. Syndecan-4 is one of the four vertebrate syndecans and has a molecular weight of ~20 kDa. Syndecans are the best-characterized plasma membrane proteoglycans. Their intracellular domain of membrane-spanning core protein interacts with actin cytoskeleton and signaling molecules in the cell cortex. Syndecans are normally found on the cell surface of fibroblasts and epithelial cells. Syndecans interact with fibronectin on the cell surface, cytoskeletal and signaling proteins inside the cell to modulate the function of integrin in cell-matrix adhesion. Also, syndecans bind to FGFs and bring them to the FGF receptor on the same cell. As a co-receptor or regulator, mutated certain proteoglycans could cause severe developmental defects, like disordered distribution or inactivation of signaling molecules.

43 kDa receptor-associated protein of the synapse (rapsyn) is a protein that in humans is encoded by the RAPSN gene.

Syndecan-3 is a protein that in humans is encoded by the SDC3 gene.

Glypican-1 (GPC1) is a protein that in humans is encoded by the GPC1 gene. GPC1 is encoded by human GPC1 gene located at 2q37.3. GPC1 contains 558 amino acids with three predicted heparan sulfate chains.

Acetylcholine receptor subunit beta is a protein that in humans is encoded by the CHRNB1 gene.
Neuromuscular junction disease is a medical condition where the normal conduction through the neuromuscular junction fails to function correctly.
The transforming growth factor beta (TGFβ) receptors are a family of serine/threonine kinase receptors involved in TGF beta signaling pathway. These receptors bind growth factor and cytokine signaling proteins in the TGF-beta family such as TGFβs, bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), activin and inhibin, myostatin, anti-Müllerian hormone (AMH), and NODAL.