
An acetylcholine receptor (abbreviated AChR) or a cholinergic receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.


An acetylcholine receptor (abbreviated AChR) or a cholinergic receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.
Like other transmembrane receptors, acetylcholine receptors are classified according to their "pharmacology," or according to their relative affinities and sensitivities to different molecules. Although all acetylcholine receptors, by definition, respond to acetylcholine, they respond to other molecules as well.
Nicotinic and muscarinic are two main kinds of "cholinergic" receptors.
Molecular biology has shown that the nicotinic and muscarinic receptors belong to distinct protein superfamilies. Nicotinic receptors are of two types: Nm and Nn. Nm [1] is located in the neuromuscular junction which causes the contraction of skeletal muscles by way of end-plate potential (EPPs). Nn causes depolarization in autonomic ganglia resulting in post ganglionic impulse. Nicotinic receptors cause the release of catecholamine from the adrenal medulla, and also site specific excitation or inhibition in brain. Both Nm and Nn are Na+ and Ca2+ channel linked but Nn is also linked with an extra K+ channel.
The nAChRs are ligand -gated ion channels, and, like other members of the "cys-loop" ligand-gated ion channel superfamily, are composed of five protein subunits symmetrically arranged like staves around a barrel. The subunit composition is highly variable across different tissues. Each subunit contains four regions which span the membrane and consist of approximately 20 amino acids. Region II which sits closest to the pore lumen, forms the pore lining.
Binding of acetylcholine to the N termini of each of the two alpha subunits results in the 15° rotation of all M2 helices. [2] The cytoplasm side of the nAChR receptor has rings of high negative charge that determine the specific cation specificity of the receptor and remove the hydration shell often formed by ions in aqueous solution. In the intermediate region of the receptor, within the pore lumen, valine and leucine residues (Val 255 and Leu 251) define a hydrophobic region through which the dehydrated ion must pass. [3]
The nAChR is found at the edges of junctional folds at the neuromuscular junction on the postsynaptic side; it is activated by acetylcholine release across the synapse. The diffusion of Na+ and K+ across the receptor causes depolarization, the end-plate potential, that opens voltage-gated sodium channels, which allows for firing of the action potential and potentially muscular contraction.
In contrast, the mAChRs are not ion channels, but belong instead to the superfamily of G-protein-coupled receptors that activate other ionic channels via a second messenger cascade. The muscarine cholinergic receptor activates a G-protein when bound to extracellular ACh. The alpha subunit of the G-protein activates guanylate cyclase (inhibiting the effects of intracellular cAMP) while the beta-gamma subunit activates the K-channels and therefore hyperpolarize the cell. This causes a decrease in cardiac activity.
ACh receptors are related to GABA, glycine, and 5-HT3 receptors and their similar protein sequence and gene structure strongly suggest that they evolved from a common ancestral receptor. [4] In fact, relatively minor mutations, such as a change in 3 amino acids in many of these receptors can convert a cation-selective channel to an anion-selective channel gated by acetylcholine, showing that even fundamental properties can relatively easily change in evolution. [5]
Acetylcholine receptor modulators can be classified by which receptor subtypes they act on:
| Drug | Nm | Nn | M1 | M2 | M3 |
|---|---|---|---|---|---|
| ACh, Carbachol, Methacholine, AChEI (Physostigmine, Galantamine, Neostigmine, Pyridostigmine) | + | + | + | + | + |
| Nicotine, Varenicline | + | + | |||
| Succinylcholine | +/- | ||||
| Atracurium, Vecuronium, Tubocurarine, Pancuronium | - | ||||
| Epibatidine, DMPP | + | ||||
| Trimethaphan, Mecamylamine, Bupropion, Dextromethorphan, Hexamethonium | - | ||||
| Muscarine, Oxotremorine, Bethanechol, Pilocarpine | + | + | + | ||
| Atropine, Tolterodine, Oxybutynin | - | - | - | ||
| Vedaclidine, Talsaclidine, Xanomeline, Ipratropium | + | ||||
| Pirenzepine, Telenzepine | - | ||||
| Methoctramine | - | ||||
| Darifenacin, 4-DAMP, Solifenacin | - |
Nicotinic acetylcholine receptors can be blocked by curare, hexamethonium and toxins present in the venoms of snakes and shellfishes, like α-bungarotoxin. Drugs such as the neuromuscular blocking agents bind reversibly to the nicotinic receptors in the neuromuscular junction and are used routinely in anaesthesia. Nicotinic receptors are the primary mediator of the effects of nicotine. In myasthenia gravis, the receptor at the neuromuscular junction is targeted by antibodies, leading to muscle weakness.
Muscarinic acetylcholine receptors can be blocked by the drugs atropine and scopolamine.
Congenital myasthenic syndrome (CMS) is an inherited neuromuscular disorder caused by defects of several types at the neuromuscular junction. Postsynaptic defects are the most frequent cause of CMS and often result in abnormalities in nicotinic acetylcholine receptors. The majority of mutations causing CMS are found in the AChR subunits genes. [6]
Out of all mutations associated with CMS, more than half are mutations in one of the four genes encoding the adult acetylcholine receptor subunits. Mutations of the AChR often result in endplate deficiency. Most of the mutations of the AChR are mutations of the CHRNE gene with mutations encoding for the Alpha5 Nicotinic Acetylcholine Receptor cause increased susceptibility to addiction. The CHRNE gene codes for the epsilon subunit of the AChR. Most mutations are autosomal recessive loss-of-function mutations and as a result there is endplate AChR deficiency. CHRNE is associated with changing the kinetic properties of the AChR. [7] One type of mutation of the epsilon subunit of the AChR introduces an Arg into the binding site at the α/ε subunit interface of the receptor. The addition of a cationic Arg into the anionic environment of the AChR binding site greatly reduces the kinetic properties of the receptor. The result of the newly introduced ARG is a 30-fold reduction of agonist affinity, 75-fold reduction of gating efficiency, and an extremely weakened channel opening probability. This type of mutation results in an extremely fatal form of CMS. [8]

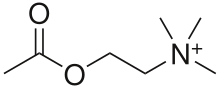
Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic.

A neurotransmitter receptor is a membrane receptor protein that is activated by a neurotransmitter. Chemicals on the outside of the cell, such as a neurotransmitter, can bump into the cell's membrane, in which there are receptors. If a neurotransmitter bumps into its corresponding receptor, they will bind and can trigger other events to occur inside the cell. Therefore, a membrane receptor is part of the molecular machinery that allows cells to communicate with one another. A neurotransmitter receptor is a class of receptors that specifically binds with neurotransmitters as opposed to other molecules.

A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.
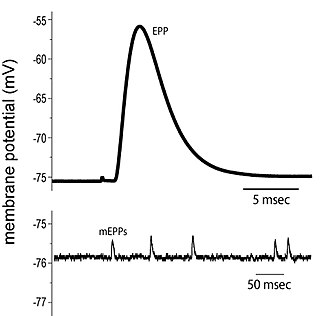
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.

Jean-Pierre Changeux is a French neuroscientist known for his research in several fields of biology, from the structure and function of proteins, to the early development of the nervous system up to cognitive functions. Although being famous in biological sciences for the MWC model, the identification and purification of the nicotinic acetylcholine receptor and the theory of epigenesis by synapse selection are also notable scientific achievements. Changeux is known by the non-scientific public for his ideas regarding the connection between mind and physical brain. As put forth in his book, Conversations on Mind, Matter and Mathematics, Changeux strongly supports the view that the nervous system functions in a projective rather than reactive style and that interaction with the environment, rather than being instructive, results in the selection amongst a diversity of preexisting internal representations.
α-Bungarotoxin is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.
The Cys-loop ligand-gated ion channel superfamily is composed of nicotinic acetylcholine, GABAA, GABAA-ρ, glycine, 5-HT3, and zinc-activated (ZAC) receptors. These receptors are composed of five protein subunits which form a pentameric arrangement around a central pore. There are usually 2 alpha subunits and 3 other beta, gamma, or delta subunits (some consist of 5 alpha subunits). The name of the family refers to a characteristic loop formed by 13 highly conserved amino acids between two cysteine (Cys) residues, which form a disulfide bond near the N-terminal extracellular domain.
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine.
Congenital myasthenic syndrome (CMS) is an inherited neuromuscular disorder caused by defects of several types at the neuromuscular junction. The effects of the disease are similar to Lambert-Eaton Syndrome and myasthenia gravis, the difference being that CMS is not an autoimmune disorder. There are only 600 known family cases of this disorder and it is estimated that its overall frequency in the human population is 1 in 200,000.

43 kDa receptor-associated protein of the synapse (rapsyn) is a protein that in humans is encoded by the RAPSN gene.

Neuronal acetylcholine receptor subunit alpha-7, also known as nAChRα7, is a protein that in humans is encoded by the CHRNA7 gene. The protein encoded by this gene is a subunit of certain nicotinic acetylcholine receptors (nAchR).

Neuronal acetylcholine receptor subunit alpha-4, also known as nAChRα4, is a protein that in humans is encoded by the CHRNA4 gene. The protein encoded by this gene is a subunit of certain nicotinic acetylcholine receptors (nAChR). Alpha4-containing nAChRs appear to play a crucial role in the addictive response to nicotine.

Neuronal acetylcholine receptor subunit beta-2 is a protein that in humans is encoded by the CHRNB2 gene.

Acetylcholine receptor subunit epsilon is a protein that in humans is encoded by the CHRNE gene.

Neuronal acetylcholine receptor subunit alpha-1, also known as nAChRα1, is a protein that in humans is encoded by the CHRNA1 gene. The protein encoded by this gene is a subunit of certain nicotinic acetylcholine receptors (nAchR).

Acetylcholine receptor subunit delta is a protein that in humans is encoded by the CHRND gene.

Acetylcholine receptor subunit beta is a protein that in humans is encoded by the CHRNB1 gene.

Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).