5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Pindolol, sold under the brand name Visken among others, is a nonselective beta blocker which is used in the treatment of hypertension. It is also an antagonist of the serotonin 5-HT1A receptor, preferentially blocking inhibitory 5-HT1A autoreceptors, and has been researched as an add-on therapy to various antidepressants, such as clomipramine and the selective serotonin reuptake inhibitors (SSRIs), in the treatment of depression and obsessive-compulsive disorder.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

Mianserin, sold under the brand name Tolvon among others, is an atypical antidepressant that is used primarily in the treatment of depression in Europe and elsewhere in the world. It is a tetracyclic antidepressant (TeCA). Mianserin is closely related to mirtazapine, both chemically and in terms of its actions and effects, although there are significant differences between the two drugs.
The 5-HT2C receptor is a subtype of the 5-HT2 receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, it is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. HTR2C denotes the human gene encoding for the receptor, that in humans is located on the X chromosome. As males have one copy of the gene and females have one of the two copies of the gene repressed, polymorphisms at this receptor can affect the two sexes to differing extent.

5-Hydroxytryptamine receptor 4 is a protein that in humans is encoded by the HTR4 gene.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

5-hydroxytryptamine (serotonin) receptor 1D, also known as HTR1D, is a 5-HT receptor, but also denotes the human gene encoding it. 5-HT1D acts on the central nervous system, and affects locomotion and anxiety. It also induces vasoconstriction in the brain.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

5-Hydroxytryptamine (serotonin) receptor 5A, also known as HTR5A, is a protein that in humans is encoded by the HTR5A gene. Agonists and antagonists for 5-HT receptors, as well as serotonin uptake inhibitors, present promnesic (memory-promoting) and/or anti-amnesic effects under different conditions, and 5-HT receptors are also associated with neural changes.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.

5-Carboxamidotryptamine (5-CT) is a tryptamine derivative closely related to the neurotransmitter serotonin.
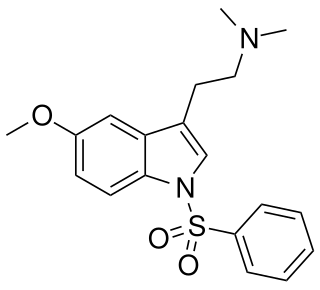
MS-245 is a tryptamine derivative used in scientific research. It acts as a selective 5-HT6 receptor antagonist with a Ki of 2.3 nM, and was derived through structure-activity relationship development of the selective 5-HT6 agonist EMDT. It has been used as a lead compound for further development of tryptamine-derived 5-HT6 antagonists. In animal studies it has been shown to boost the activity of, but not substitute for, both amphetamine and nicotine.

SB-269970 is a drug and research chemical developed by GlaxoSmithKline used in scientific studies. It is believed to act as a selective 5-HT7 receptor antagonist (EC50 = 1.25 nM) (or possibly inverse agonist). A subsequent study in guinea pig at a concentration of 10 μM showed that it also blocks the α2-adrenergic receptor. The large difference in test concentrations however confirms the selectivity of SB-269970 for the 5-HT7 receptor.
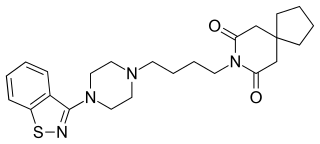
Tiospirone (BMY-13,859), also sometimes called tiaspirone or tiosperone, is an atypical antipsychotic of the azapirone class. It was investigated as a treatment for schizophrenia in the late 1980s and was found to have an effectiveness equivalent to those of typical antipsychotics in clinical trials but without causing extrapyramidal side effects. However, development was halted and it was not marketed. Perospirone, another azapirone derivative with antipsychotic properties, was synthesized and assayed several years after tiospirone. It was found to be both more potent and more selective in comparison and was commercialized instead.
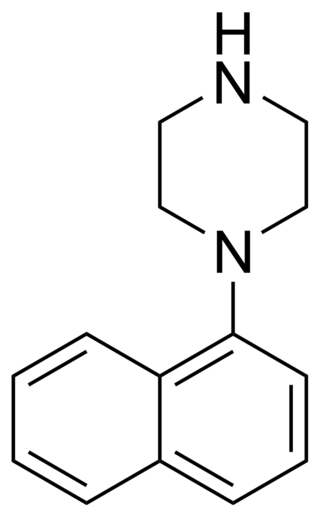
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.
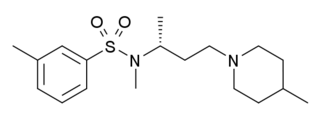
SB-258719 is a drug developed by GlaxoSmithKline which acts as a selective 5-HT7 receptor partial inverse agonist, and was the first such ligand identified for 5-HT7. Its use in research has mainly been in demonstrating the potential use for 5-HT7 agonists as potential novel analgesics, due to the ability of SB-258719 to block the analgesic effects of a variety of 5-HT7 agonists across several different testing models.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.
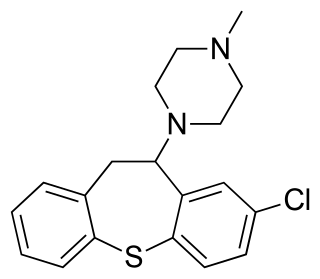
Clorotepine, also known as octoclothepin or octoclothepine, is an antipsychotic of the tricyclic group which was derived from perathiepin in 1965 and marketed in the Czech Republic by Spofa in or around 1971 for the treatment of schizophrenic psychosis.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.