
Mirtazapine, sold under the brand name Remeron among others, is an atypical tetracyclic antidepressant, and as such is used primarily to treat depression. Its effects may take up to four weeks but can also manifest as early as one to two weeks. It is often used in cases of depression complicated by anxiety or insomnia. The effectiveness of mirtazapine is comparable to other commonly prescribed antidepressants. It is taken by mouth.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Tiotixene, or thiothixene is a typical antipsychotic agent currently sold under the brand name Navane which is predominantly utilised to treat acute and chronic schizophrenia. Beyond its primary indication, it can exhibit a variety of effects common to neuroleptic drugs including anxiolytic, anti-depressive, and anti-aggressive properties.

Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression. It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively. The drug is described as an atypical or "second-generation" TCA because, unlike other TCAs, it seems to be a fairly weak monoamine reuptake inhibitor. Similarly to other TCAs, however, trimipramine does have antihistamine, antiserotonergic, antiadrenergic, antidopaminergic, and anticholinergic activities.

Trazodone, sold under many brand names, is an antidepressant medication, used to treat major depressive disorder, anxiety disorders, and insomnia. It is a phenylpiperazine compound of the serotonin antagonist and reuptake inhibitor (SARI) class. The medication is taken orally.

Chlorprothixene, sold under the brand name Truxal among others, is a typical antipsychotic of the thioxanthene group.

Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent. It was chiefly investigated as a drug to treat insomnia, especially to enhance sleep quality by significantly increasing slow wave sleep by virtue of potent and concomitant 5HT2a and 5HT2c antagonism

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.
A serotonin antagonist, or serotonin receptor antagonist, is a drug used to inhibit the action of serotonin and serotonergic drugs at serotonin (5-HT) receptors.

Altanserin is a compound that binds to the 5-HT2A receptor. Labeled with the isotope fluorine-18 it is used as a radioligand in positron emission tomography (PET) studies of the brain, i.e., studies of the 5-HT2A neuroreceptors. Besides human neuroimaging studies altanserin has also been used in the study of rats.

Eplivanserin was an experimental drug for the treatment of insomnia which was being developed by Sanofi Aventis.
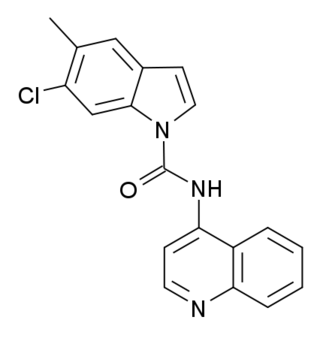
SB-215505 is a drug which acts as a potent and selective antagonist at the serotonin 5-HT2B receptor, with good selectivity over the related 5-HT2A and 5-HT2C receptors. It is used in scientific research into the function of the 5-HT2 family of receptors, especially to study the role of 5-HT2B receptors in the heart, and to distinguish 5-HT2B-mediated responses from those produced by 5-HT2A or 5-HT2C.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

Clocapramine, also known as 3-chlorocarpipramine, is an atypical antipsychotic of the iminostilbene class which was introduced in Japan in 1974 by Yoshitomi for the treatment of schizophrenia. In addition to psychosis, clocapramine has also been used to augment antidepressants in the treatment of anxiety and panic.

Glemanserin (INN) is a drug which acts as a potent and selective 5-HT2A receptor antagonist. The first truly selective 5-HT2A ligand to be discovered, glemanserin resulted in the development of the widely used and even more potent and selective 5-HT2A receptor antagonist volinanserin (MDL-100,907), which is a fluorinated analogue. Though it was largely superseded in scientific research by volinanserin, glemanserin was investigated clinically for the treatment of generalized anxiety disorder. However, it was ultimately found to be ineffective and was not marketed.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.
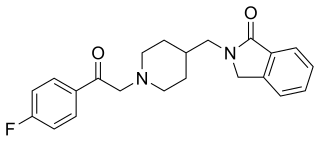
Roluperidone (former developmental code names MIN-101, CYR-101, MT-210) is a 5-HT2A and σ2 receptor antagonist under development by Minerva Neurosciences for the treatment of schizophrenia. One of its metabolites also has some affinity for the H1 receptor. Pre-clinical findings provide evidence of the effect of roluperidone on Brain-Derived Neurotrophic Factor (“BDNF”), which has been associated with neurogenesis, neuroplasticity, neuroprotection, synapse regulation, learning and memory.

AC-90179 is a piperidine derivative which acts as an inverse agonist at the 5-HT2A serotonin receptor and an antagonist at 5-HT2C. It was developed as a potential antipsychotic but was not pursued for medical applications due to poor oral bioavailability; however, it continues to be used as a tool compound in pharmacological research.


















