
Lysergic acid diethylamide, commonly known as LSD, is a potent psychedelic drug that intensifies thoughts, emotions, and sensory perception. Often referred to as acid or lucy, LSD can cause mystical, spiritual, or religious experiences. At higher doses, it primarily induces visual and auditory hallucinations. While LSD does not cause physical addiction, it can lead to adverse psychological reactions, such as anxiety, paranoia, and delusions. Additionally, it may trigger "flashbacks," also known as hallucinogen persisting perception disorder, where individuals experience persistent visual distortions after use.

Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary mental states and a perceived "expansion of consciousness". Also referred to as classic hallucinogens or serotonergic hallucinogens, the term psychedelic is sometimes used more broadly to include various types of hallucinogens, such as those which are atypical or adjacent to psychedelia like salvia and MDMA, respectively.
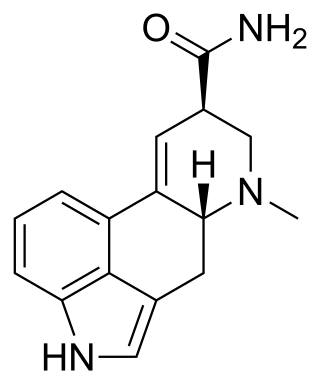
Ergine, also known as d-lysergic acid amide (LSA) and d-lysergamide, is an ergoline alkaloid that occurs in various species of vines of the Convolvulaceae and some species of fungi. The psychedelic properties in the seeds of ololiuhqui, Hawaiian baby woodrose and morning glories have been linked to ergine and/or isoergine, its epimer, as it is an alkaloid present in the seeds.
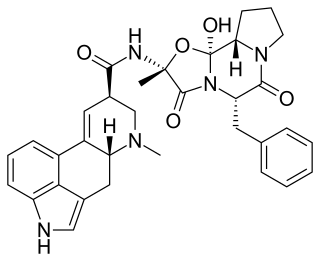
Ergotamine, sold under the brand name Ergomar among others, is an ergopeptine and part of the ergot family of alkaloids; it is structurally and biochemically closely related to ergoline. It is structurally similar to several neurotransmitters, and it acts as a vasoconstrictor. It is used for acute migraines, sometimes with caffeine as the combination ergotamine/caffeine.

5-Hydroxytryptophan (5-HTP), used medically as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.
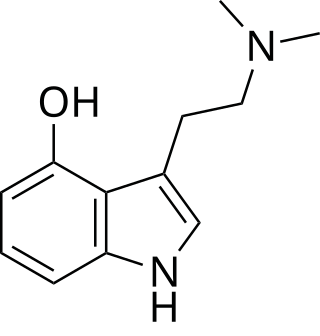
Psilocin, also known as 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT), is a substituted tryptamine alkaloid and a serotonergic psychedelic. It is present in most psychedelic mushrooms together with its phosphorylated counterpart psilocybin. Psilocin is a Schedule I drug under the Convention on Psychotropic Substances. Acting on the serotonin 5-HT2A receptors, psilocin's psychedelic effects are directly correlated with the drug's occupancy at these receptor sites. The subjective mind-altering effects of psilocin are highly variable and are said to resemble those of lysergic acid diethylamide (LSD) and N,N-dimethyltryptamine (DMT).

Amides of lysergic acid are collectively known as lysergamides, and include a number of compounds with potent agonist and/or antagonist activity at various serotonin and dopamine receptors. Lysergamides contain an embedded tryptamine structure, and as a result can produce similar, often psychedelic, effects to those of the true tryptamines.

Ergometrine, also known as ergonovine and sold under the brand names Ergotrate, Ergostat, and Syntometrine among others, is a medication used to cause contractions of the uterus to treat heavy vaginal bleeding after childbirth. It can be used either by mouth, by injection into a muscle, or injection into a vein. It begins working within 15 minutes when taken by mouth and is faster in onset when used by injection. Effects last between 45 and 180 minutes.

Methylergometrine, also known as methylergonovine and sold under the brand name Methergine, is a medication of the ergoline and lysergamide groups which is used as an oxytocic in obstetrics and in the treatment of migraine. It reportedly produces psychedelic effects similar to those of lysergic acid diethylamide (LSD) at high doses.
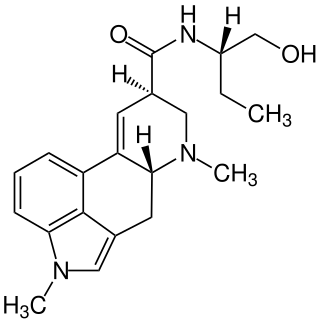
Methysergide, sold under the brand names Deseril and Sansert, is a monoaminergic medication of the ergoline and lysergamide groups which is used in the prophylaxis and treatment of migraine and cluster headaches. It has been withdrawn from the market in the United States and Canada due to adverse effects. It is taken by mouth.
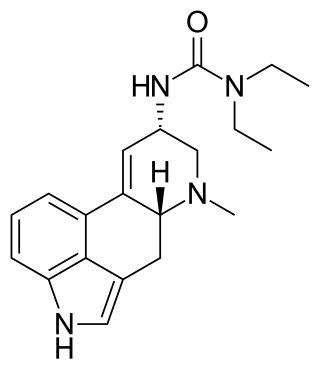
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

Lysergic acid 2,4-dimethylazetidide (LA-SS-Az, LSZ) is an analog of LSD developed by the team led by David E. Nichols at Purdue University. It was developed as a rigid analog of LSD with the diethylamide group constrained into an azetidine ring in order to map the binding site at the 5-HT2A receptor. There are three possible stereoisomers around the azetidine ring, with the (S,S)-(+) isomer being the most active, slightly more potent than LSD itself in drug discrimination tests using trained rats.

25B-NBOMe is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor. Duration of effects lasts about 3–10 hours, although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses. Recently, Custodio et al. (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

1-Methylpsilocin (developmental code names CMY, CMY-16) is a tryptamine derivative developed by Sandoz which acts as a selective agonist of the serotonin 5-HT2C receptor (IC50Tooltip half-maximal inhibitory concentration of 12 nM, vs. 633 nM at 5-HT2A), and an inverse agonist at 5-HT2B (Ki of 38 nM). While 1-methylpsilocin does have higher affinity for 5-HT2C than 5-HT2A, it does produce a head-twitch response in mice that is dependent on 5-HT2A, so it is not entirely free of effects on 5-HT2Ain vivo. In contrast to psilocin, 1-methylpsilocin did not activate 5-HT1A receptors in mice.

Pirenperone (INNTooltip International Nonproprietary Name, USANTooltip United States Adopted Name, BANTooltip British Approved Name; developmental code names R-47456, R-50656) is a serotonin receptor antagonist described as an antipsychotic and tranquilizer which was never marketed. It is a relatively selective antagonist of the serotonin 5-HT2 receptors and has been used in scientific research to study the serotonin system. In the 1980s, the drug was found to block the effects of the lysergic acid diethylamide (LSD) in animals, and along with ketanserin, led to the elucidation of the 5-HT2A receptor as the biological mediator of the effects of serotonergic psychedelics.
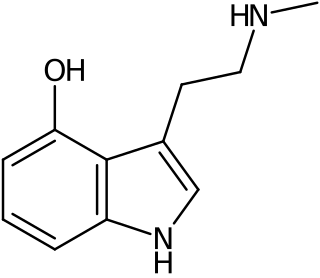
Norpsilocin, also known as 4-hydroxy-N-methyltryptamine (4-HO-NMT), is a tryptamine alkaloid recently discovered in 2017 in the psychedelic mushroom Psilocybe cubensis. It is hypothesized to be a dephosphorylated metabolite of baeocystin.




















