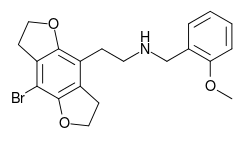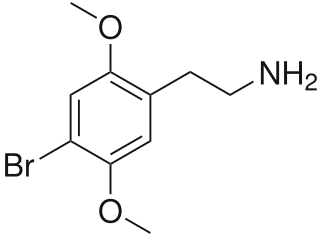
2C-B (4-bromo-2,5-dimethoxyphenethylamine), also known as Nexus, is a synthetic psychedelic drug of the 2C family, mainly used as a recreational drug. The substance was first synthesized by Alexander Shulgin in 1974, and gained an initial reputation for potential psychotherapeutic use, but its use has been limited to mainly recreational use. To date, there is limited scientific information regarding the drug's pharmacokinetics and pharmacological effects in humans. The existing studies primarily classify 2C-B as a stimulant and hallucinogen, and less commonly an entactogen and empathogen.
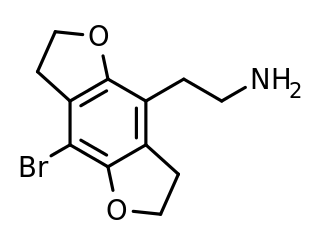
2C-B-FLY is a psychedelic phenethylamine and designer drug of the 2C family. It was first synthesized in 1996 by Aaron Monte, Professor of Chemistry at UW-La Crosse.
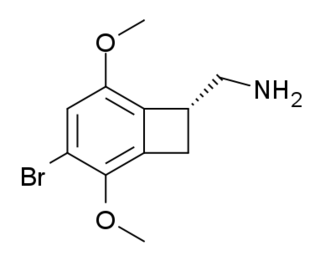
TCB-2 is a hallucinogen discovered in 2006 by Thomas McLean working in the lab of David Nichols at Purdue University. It is a conformationally-restricted derivative of the phenethylamine 2C-B, also a hallucinogen, and acts as a potent agonist for the 5-HT2A and 5-HT2C receptors with a Ki of 0.26 nM at the human 5-HT2A receptor. In drug-substitution experiments in rats, TCB-2 was found to be of similar potency to both LSD and Bromo-DragonFLY, ranking it among the most potent phenethylamine hallucinogens yet discovered. This high potency and selectivity has made TCB-2 useful for distinguishing 5-HT2A mediated responses from those produced by other similar receptors. TCB-2 has similar but not identical effects in animals to related phenethylamine hallucinogens such as DOI, and has been used for studying how the function of the 5-HT2A receptor differs from that of other serotonin receptors in a number of animal models, such as studies of cocaine addiction and neuropathic pain.

25I-NBOH is a derivative of the phenethylamine-derived hallucinogen 2C-I that was discovered in 2006 by a team at Purdue University.

25I-NBOMe, also known as Smiles, or N-Bomb, is a novel synthetic psychoactive substance with strong hallucinogenic properties, synthesized in 2003 for research purposes. Since 2010, it has circulated in the recreational drug scene, often misrepresented as LSD.

2CBCB-NBOMe (NBOMe-TCB-2) is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2007 at Purdue University as part of the ongoing research program of the team led by David Nichols focusing on the mapping of the specific amino acid residues responsible for ligand binding to the 5HT2A receptor. 2CBCB-NBOMe acts as a potent and selective agonist for the 5-HT2A and 5-HT2C receptors, with a Ki of 0.27 nM at the human 5-HT2A receptor, a similar potency to other agonists such as TCB-2, NBOMe-2C-I and Bromo-DragonFLY.

25I-NBMD is a derivative of the phenethylamine hallucinogen 2C-I, discovered in 2006 by a team at Purdue University led by David Nichols. It acts as a potent partial agonist for the 5HT2A receptor with a Ki of 0.049 nM at the human 5HT2A receptor. The corresponding 4-bromo analogue 25B-NBMD has been used for molecular dynamics studies on the shape of the 5-HT2A receptor.

25B-NBOMe is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor. Duration of effects lasts about 3–10 hours, although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses. Recently, Custodio et al. (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.

2CB-Ind is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, discovered in 1974 by Alexander Shulgin. It acts as a moderately potent and selective agonist for the 5-HT2A and 5-HT2C receptors, but unlike the corresponding benzocyclobutene derivative TCB-2 which is considerably more potent than the parent compound 2C-B, 2CB-Ind is several times weaker, with racemic 2CB-Ind having a Ki of 47nM at the human 5-HT2A receptor, only slightly more potent than the mescaline analogue (R)-jimscaline.
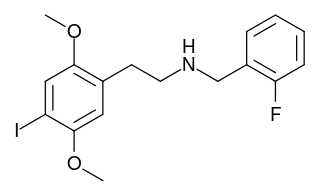
25I-NBF is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a highly potent partial agonist for the human 5-HT2A receptor, with bias towards the β-arrestin 2 coupled signalling pathway. It has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET).

25TFM-NBOMe is a derivative of the phenethylamine hallucinogen 2C-TFM, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent partial agonist for the 5-HT2A receptor, though its relative potency is disputed, with some studies finding it to be of lower potency than 25I-NBOMe, while others show it to be of similar or higher potency, possibly because of differences in the assay used. 2C-TFM-NB2OMe can be taken to produce psychedelic effects similar to 2C-I-NB2OMe and 2C-D-NB2OMe.
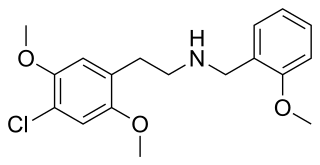
25C-NBOMe is a psychedelic drug and derivative of the psychedelic phenethylamine 2C-C. 25C-NBOMe appeared on online vendor sites in 2010 but was not reported in the literature until 2011. It acts as a potent agonist of the 5-HT2A receptor, and has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET). Multiple deaths have occurred from usage of 25C-NBOMe due to the ease of accidental overdose. The long-term toxic effects of the drug have not been researched.
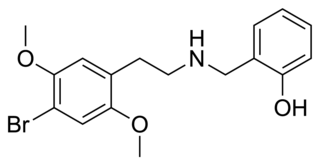
25B-NBOH is a derivative of the phenethylamine derived hallucinogen 2C-B which has been sold as a designer drug. It acts as a potent serotonin receptor agonist with similar affinity to the better-known compound 25B-NBOMe at 5-HT2A and 5-HT2C receptors with pKis values of 8.3 and 9.4, respectively.

NBOMe-mescaline or mescaline-NBOMe is a synthetic substituted phenethylamine. It is a partial agonist of serotonin receptors with a 5-HT2A pKi originally reported as 7.3, though more modern techniques assayed it as 140nM at 5-HT2A and 640nM at 5-HT2C, making it one of the least potent compounds among the N-benzyl phenethylamines.
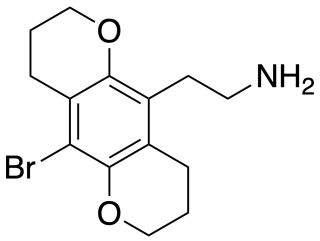
2C-B-BUTTERFLY is a conformationally-restricted derivative of the phenethylamine hallucinogen 2C-B, which was discovered in 1999 by Michael S. Whiteside and Aaron Monte. It is a ring-expanded homologue of the better known compound 2C-B-FLY, and has similar properties as an agonist for serotonin receptors, but with more selectivity for 5-HT2C over 5-HT2A.

25I-NB34MD (NB34MD-2C-I) is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a potent partial agonist for the human 5-HT2A receptor, and presumably has similar properties to 2C-I. It has a binding affinity of 0.67nM at the human 5-HT2A receptor, making it several times weaker than its positional isomer 25I-NBMD and a similar potency to 25I-NBF.
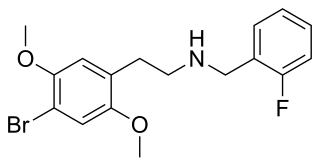
25B-NBF is a derivative of the phenethylamine hallucinogen 2C-B, which acts as a highly potent partial agonist for the human 5-HT2A receptor.

25C-NBF is a derivative of the phenethylamine hallucinogen 2C-C, which acts as a highly potent partial agonist for the human 5-HT2A receptor.

25H-NBOMe (NBOMe-2C-H) is a derivative of the phenethylamine hallucinogen 2C-H, which acts as a highly potent full agonist for the human 5-HT2A receptor.

The 25-NB (25x-NBx) series, sometimes alternatively referred to as the NBOMe compounds, is a family of serotonergic psychedelics. They are substituted phenethylamines and were derived from the 2C family. They act as selective agonists of the serotonin 5-HT2A receptor. The 25-NB family is unique relative to other classes of psychedelics in that they are, generally speaking, extremely potent and relatively selective for the 5-HT2A receptor. Use of NBOMe series drugs has caused many deaths and hospitalisations since the drugs popularisation in the 2010s. This is primarily due to their high potency, unpredictable pharmacokinetics, and sellers passing off the compounds in the series as LSD.